|
 |
| J Korean Acad Pediatr Dent > Volume 42(4); 2015 > Article |
|
міИл°Э
л≥Є мЧ∞кµђмЭШ л™©м†БмЭА лЛ§мЦСнХЬ MTA мЮђл£М(ProRoot MTA, RetroMTA, Endocem Zr)мЧР лМАнХЬ мЬ†мєШ мєШмИШ мДЄнПђмЭШ мГЭнЩЬ놕 л∞П лґДнЩФлК•мЭД лєДкµР нПЙк∞АнХШлКФ к≤ГмЭілЛ§.
мЬ†мєШ мєШмИШмДЄнПђлКФ к∞Б мЮђл£Мл≥Дл°Ь к≤љнЩФлРЬ мЫРнШХ лФФмК§нБђл•Љ мЭімЪ©нХШмЧђ мІБм†Сл≤Х л∞П к∞Дм†Сл≤ХмЬЉл°Ь мДЄнПђ мГЭнЩЬ놕мЭД кіАм∞∞нХШмШАлЛ§. лШРнХЬ мЮђл£Мл≥Д мґФмґЬлђЉмЭД мЭімЪ©нХШмЧђ pHл•Љ мЄ°м†ХнХШмШАмЬЉл©∞, alkaline phosphatase(ALP) нЩЬмД±лПД л∞П Alizarin Red S мЧЉмГЙл≤ХмЭД нЖµнХШмЧђ мДЄнПђмЭШ лґДнЩФлК•мЭД кіАм∞∞нХШмШАлЛ§.
мІБм†Сл≤ХмЧРмДЬ мЬ†мєШ мєШмИШмДЄнПђлКФ ProRoot MTAмЩА RetroMTAмЧРмДЬ Endocem ZrмЧР лєДнХі лЖТмЭА мДЄнПђ мГЭнЩЬ놕мЭД л≥імШАмЬЉлВШ, л∞Шл©і к∞Дм†Сл≤ХмЧРмДЬлКФ Endocem ZrмЧРмДЬ лЛ§л•Є мЮђл£МмЧР лєДнХі лЖТмЭА мДЄнПђ мГЭнЩЬ놕мЭі кіАм∞∞лРШмЧИлЛ§. pHмЭШ к≤љмЪ∞ Endocem Zrк∞А лЛ§л•Є лСР мЮђл£МмЧР лєДнХі лВЃмЭА мХМмєЉл¶ђмД±мЭД лВШнГАлГИлЛ§. л™®лУ† мЮђл£МмЧРмДЬ ALP нЩЬмД±лПДлКФ лМАм°∞кµ∞мЧР лєДнХі м¶Эк∞АнХШмІА мХКмХШмЬЉл©∞, Alizarin Red S мЧЉмГЙ к≤∞к≥Љ мЬ†мєШ мєШмИШмДЄнПђмЭШ лґДнЩФлК•мЭі лМАм°∞кµ∞мЧР лєДнХі лВЃмХШлЛ§.
л≥Є мЛ§нЧШмЧРмДЬ мЮђл£Мл≥Д м∞®мЭілКФ мЮИмЧИмЬЉлВШ л™®лУ† мЮђл£МмЧРмДЬ мЦілКР м†ХлПДмЭШ мДЄнПђ лПЕмД±мЭі кіАм∞∞лРШмЧИмЬЉл©∞, мЬ†мєШ мєШмИШмДЄнПђмЭШ мГЭнЩЬ놕к≥Љ лґДнЩФлК•мЭД м¶ЭмІДмЛЬнВ§мІА л™їнХШмШАлЛ§. нХШмІАлІМ Endocem ZrмЭШ к≤љмЪ∞ ProRoot MTAлВШ RetroMTAмЧР лєДнХі лВЃмЭА мХМмєЉл¶ђмД±к≥Љ лЖТмЭА мГЭнЩЬ놕мЭД л≥імШАлЛ§.
Abstract
This study compared the in vitro cell viability and differentiation potentials of human deciduous dental pulp cells (DPCs) on mineral trioxide aggregate (MTA)-like products (ProRoot MTA, RetroMTA and Endocem Zr).
The experimental materials were prepared as circular discs, which were used to test the effects of the materials on the viability of human DPCs when placed in direct and indirect contact. Furthermore, the pH of the extracted materials was recorded, and their effect on cell differentiation potential was evaluated by evaluating the alkaline phosphatase (ALP) activity and Alizarin Red S staining of DPCs incubated with the test materials.
In direct contact, the cell viability of human DPCs was higher with ProRoot MTA and RetroMTA than with Endocem Zr. However, when in indirect contact, the cell viability of human DPCs was generally higher in Endocem Zr than in ProRoot MTA and Retro MTA. With respect to pH, the alkalinity was lower for Endocem Zr than for the other test materials. The ALP activities of the cells were not enhanced by any of the experimental materials. Alizarin Red S staining of the tested human DPCs revealed that their differentiation potential was lower than for cells incubated with osteogenic induction medium.
While there were differences in the responses of the human DPCs to the test materials, all displayed degrees of cytotoxicity and were unable to enhance either the viability or differentiation of human DPCs. However, Endocem Zr exhibited better cell viability and was less alkaline than the other test materials.
Mineral trioxide aggregate (MTA) has been used in numerous clinical applications in the field of endodontics since it received approval from the US Food and Drug Administration in 1998. Its excellence in biocompatibility and sealing ability has led to its use in endodontics for a variety of treatments such as apexification, pulpotomy, and pulp capping [1]. Recent clinical studies have demonstrated the sealing ability and biologic responses of MTA in pulpotomy and pulp capping in permanent [2] and deciduous [3,4] teeth. In both tooth types, MTA has produced significantly more favorable results with regard to efficacy and stability as a pulp treatment medicament compared to the conventionally used calcium hydroxide [Ca(OH)2] [5-8].
The success of pulp treatment can be attributed to the degree of initial cytotoxicity and the effect on pulpal inflammation associated with pulp capping/pulpotomy materials, since they come into direct contact with the pulp tissue. Previous in vivo studies have found that MTA induces less inflammation in the pulp tissue than Ca(OH)2 [9,10]. However, yet other researchers have reported that after contact with pulp tissue, MTA also induces tissue necrosis at the regions nearest to the hard-tissue bridge [11,12]. A detailed in vitro study of the initial cytotoxicity associated with MTA is needed to unveil the effects of such an inflammatory reaction to the treatment outcome.
The results of recent studies have been controversial regarding the cytotoxicity of MTA. Some have demonstrated that pulp cells cultured on MTA have a higher survival rate than those cultured on other endodontic materials [13], and enhance cell differentiation into various hard-tissue formation precursors such as dentin sialoprotein, osteocalcin, and alkaline phosphatase (ALP) [14]. However, others studies have found that MTA reduces the cell-survival rate when it comes into contact with pulp cells [15]. Moreover, the most recent studies are limited to ProRoot MTA and to human pulp cells from permanent teeth.
The increasing use of MTA in deciduous teeth and the differences in the responses to this material of cells from permanent and deciduous teeth stresses the need for in vitro studies using deciduous dental pulp cells (DPCs). In addition, while there are numerous studies on ProRoot MTA, there is a dearth of in vitro reports regarding newly developed MTA materials based on those previous studies. In particular, pulp treatment materials that have been developed and produced in South Korea, such as Retro MTA and Endocem Zr, are being used clinically without experimental evidence of their efficacy and stability.
This in vitro study addressed the previous controversial reports of the initial cytotoxicity of MTA on human deciduous DPCs by comparing the cellular responses of three commercially available MTA-like products [ProRoot MTA, Retro MTA, and Endocem Zr] and Intermediate Restorative Material (IRM). The primary aim was to determine the effect of these materials on the proliferation and differentiation of human deciduous DPCs.
Human dental pulp stem cells were obtained from the deciduous teeth of six children (aged 2-5 years; two males and four females) under approved guidelines set by the Institutional Review Board of the Dental Hospital (#2-2013-0016). Pulp tissue was extirpated from the decayed deciduous teeth using a barbed broach, treated with collagenase type I (3 mg/ml; Invitrogen, Carlsbad, CA, USA) and dispase (4 mg/ml; Invitrogen) for 20 minutes at 37вДГ, and then filtered through a 70-ќЉm cell strainer (BD Falcon, Lincoln Park, NJ, USA). The isolated DPCs were cultured in alpha-minimum essential medium (ќ±-MEM; Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin (Invitrogen), 100 ќЉg/ml streptomycin (Invitrogen), and 2 mM L-glutamine (Invitrogen) at 37вДГ in an incubator under 5% CO2 and 100% humidity. The isolated stem cells originating from individual teeth were blended at passage 2, and passages 3-5 were used for further experiments.
The MTA-like products used in this study were ProRoot MTA¬Ѓ (Dentsply Tulsa Dental, Tulsa, OK, USA), Retro MTA¬Ѓ (BioMTA, Seoul, Korea), Endocem Zr¬Ѓ (Maruchi, Wonju, Korea), and IRM¬Ѓ (Dentsply). These materials were prepared according to the manufacturerвАЩs instructions. The mixtures of materials were packed into rubber molds (8 mm in diameter and 1 mm thick), covered with overhead projector (OHP) film (CG6000, 3M Korea, Seoul, Korea), and allowed to set in an incubator for 1 day under conditions of 100% humidity and 37вДГ. After removing the OHP film, the mixtures were replaced into the incubator for one more day. The set discs were removed from their rubber molds and sterilized under ethylene oxide.
The discs of experimental material were placed into 50-ml conical tubes (SPL, Pocheon, Korea) and extracted with growth medium or osteogenic induction medium in an incubator for 3 days at 37вДГ under 5% CO2 and 100% humidity, in accordance with ISO 10993-12:2007(8 mm √Ч 1 mm, 0.42 ml). The osteogenic induction medium was made by adding osteogenic factors, 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 2 mM ќ≤-glycerophosphate (Sigma-Aldrich), and 50 ќЉM L-ascorbic acid 2-phosphate (Sigma-Aldrich) to the growth medium. Fragments were removed from the extracted media by centrifuging them at 10,000 rpm for 20 minutes and then filtering them through a cellulose acetate membrane filter (pore size 0.2 ќЉm; Advantec, Tokyo, Japan).
After extraction for 3 days with growth medium, as described above, human deciduous DPCs were seeded at a concentration of 2√Ч104 cells/well on the discs in 48-well plates (BD Falcon) for 3 days. The cells on the discs were then incubated in fixative solution containing 2% paraformaldehyde, 2% glutaraldehyde, and 0.5% calcium chloride, and then postfixed in 1% osmium tetroxide (all Sigma-Aldrich). The samples were dehydrated in a series of graded ethanols (50%, 70%, 80%, 95%, and 100%), dried, coated with a 100-nm layer of platinum, and then visualized using a scanning electron microscope (SEM; Hitachi S-3000N, Hitachi, Tokyo, Japan) at an accelerating voltage of 20.0 kV and a magnification of √Ч 500.
The viability of human DPCs from deciduous teeth was evaluated using two methods: (1) direct contact between the experimental disc and the DPCs and (2) treatment of the DPCs with extracted medium from the experimental discs. Cell viability was measured using a Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan), according to the manufacturerвАЩs instructions.
In the first experimental protocol, direct contact with the disc, extracted medium was first obtained from the discs as described above. Human DPCs were then seeded at 2√Ч104 cells/well onto the discs in 48-well plates. After 1 and 3 days, discs with cells were transferred to new 48-well plates, and growth medium and CCK-8 reagent were added to each well, and incubated at 37вДГ for 4 hours. The amount of the reduced water-soluble formazan that is produced by the activity of dehydrogenases in living cells was measured using a spectrophotometer (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm.
In the second experimental protocol, treatment of human DPCs with extracted medium from the discs, the cells were seeded at 2√Ч103 cells/well in 48-well plates. After 3 days, when the cells had reached about 50% confluency, the cell culture medium was changed to extracted growth medium diluted to concentrations of 1:1, 1:2, and 1:4. After 3 days in the extracted medium, CCK-8 reagent was added to each well and incubated at 37вДГ for 1 hour. The amount of reduced formazan produced was measured as described above.
As a control, the cells were cultured in growth medium alone for both the direct and indirect methods.
Experimental discs were extracted with ќ±-MEM (No Phenol Red; Invitrogen) for 3 days, and the extracted media were diluted to concentrations of 1:1, 1:2, and 1:4. The pH of each concentration of extracted medium was measured using a pH meter (Orion Star A211 pH Benchtop Meter, Thermo Scientific, Rockford, IL, USA). Color changes were obtained using the pH indicator thymol blue (pH 8.0-9.6; Daejung Chemicals, Siheung, Korea) and Alizarin Yellow R (pH 10.0-12.0; Sigma-Aldrich).
DPCs were seeded at 1√Ч104 cells/well into 12-well culture plates (BD Falcon). After 1 day, when the cells had reached about 30% confluency, the cell culture medium was changed to extracted growth medium diluted to a concentration of 1:4, and refreshed twice weekly thereafter. For a negative and positive controls, the cells were cultured in growth medium alone and osteogenic induction medium, respectively. After 10 days the cells were fixed with 10% neutral buffered formalin (Sigma-Aldrich) at 4вДГ for 30 minutes, washed with phosphate buffered saline (Invitrogen), and stained with ALP staining solution [100 mM Tris-HCl, pH 8.4 (Welgene, Daegu, Korea), 0.01% naphthol AS-MX phosphate (Sigma-Aldrich), and 0.06% Fast Red Violet LB salt (Sigma-Aldrich)], which represented a modification of the staining solution introduced previously [16], for 30 minutes at room temperature. The cells were washed three times with distilled water and then the color change was observed.
The ALP activity assay was performed as described elsewhere [17]. In brief, the level of ALP activity was measured using a SensoLyte¬Ѓ pNPP Alkaline Phosphatase Assay Kit (AnaSpec, Fremont, CA, USA), according to manufacturerвАЩs instructions. The supernatant of the cell lysates was used for detection of ALP activity. The absorbance was measured using a microplate spectrophotometer (Bio-Rad) at 405 nm. The measured ALP activity was normalized against total protein quantity in the supernatant of the same cell lysates using a Pierce BCA Protein Assay Kit (Thermo Scientific).
DPCs were seeded a density of 1√Ч104 cells/well in 12-well culture plates. After 1 day, when the DPCs had reached about 30% confluency, they were cultured in extracted osteogenic-induction medium diluted to a concentration of 1:4; the medium was refreshed twice weekly thereafter. For negative and positive controls, the cells were cultured in growth medium alone and osteogenic induction medium, respectively. After 3 weeks, extracellular mineralization was quantified using Alizarin Red S staining. The cells were fixed for 30 minutes with 10% neutral buffered formalin (Sigma-Aldrich) at 4вДГ and stained with 2% Alizarin Red S solution (pH 4.2; Sigma-Aldrich) for 10 minutes at room temperature. Stained mineralization nodules were observed using an inverted microscope (Leica, Bensheim, Germany). The stained nodules were quantified by incubating the cells in 10% cetylpyridinium chloride for 10 minutes under conditions of gentle agitation; the amount of extracted dye was measured using a microplate spectrophotometer (Bio-Rad) at 570 nm.
All experiments were conducted at least in triplicate. All data are presented as mean and SD values. The statistical analyses were performed with SPSS (19.0, Chicago, IL, USA). The significance of the findings for the cell viability test was determined by multiple comparison testing using ANOVA (p < 0.05) followed by the post-hoc Tukey test with Bonferroni correction (p < 0.01). For ALP activity and the Alizarin Red S extraction test, multiple comparison was performed using the Kruskal-Wallis test (p < 0.05) followed by the Mann-Whitney test with Bonferroni correction (p < 0.01).
SEM analysis was performed to explore the surface of the experimental discs before and after extraction, and to examine the morphology of the DPCs growing on the discs (Fig. 1). The surface of extracted discs (Fig. 1E-H) was uneven and rough compared to that of the nonextracted discs (Fig. 1A-D). Discs made from Endocem Zr exhibited a less porous surface than those made with ProRoot MTA and Retro MTA. Cells on the Endocem Zr, ProRoot MTA, and Retro MTA discs were spindle-shaped and adhered to the surface (Fig. 1F-H, arrows). In contrast, cells on the IRM disc were rounded, and few had adhered to the disc surface (Fig. 1E).
When in direct contact with the discs, the cell viability of experimental groups was significantly lower than in the control group. On day 1, the cell viability of the IRM and Endocem Zr groups was lower than 20%, whereas those of the ProRoot MTA and Retro MTA groups were 48% and 51%, respectively. Cell viability was lower on day 3 than on day 1 in all groups. In the IRM and Endocem Zr groups, there were very few live on the disc surface (i.e., cell viability was lower than 5%). The cell viabilities in the ProRoot MTA and Retro MTA groups were about 21% and 17%, respectively, on day 3 (Fig. 2).
With the indirect treatment with extracted medium from the disc, the cell viability in three of the experimental groups (ProRoot MTA, Retro MTA, and Endocem Zr) appeared to differ according to the concentration of the extracted medium. However, there were no living cells in the IRM group, regardless of the extracted medium concentration. At the original concentration, the three experimental groups in which live cells were found exhibited very low cell viability compared to the control group. At a concentration of 1:2, the cell viability of the Endocem Zr group had recovered to the control level, whereas that for the ProRoot MTA and Retro MTA groups was about 50% of control levels. At the 1:4 concentration, the cell viability of the ProRoot MTA and Retro MTA groups had recovered to a level close to control levels (Fig. 3).
The media extracted from the experimental discs were alkaline in all cases except IRM. The extracted media of ProRoot MTA and Retro MTA discs were particularly strongly alkaline (i.e., pH higher than 12), with no significant difference between the two groups. The pH was lower in the Endocem Zr group than in the ProRoot MTA and Retro MTA groups (Fig. 4A).
The color changes observed using the pH indicator corresponded with the pH measurements. At the 1:2 concentration, the pH of the Endocem Zr extracted medium was between 8 and 10, and that of the ProRoot MTA and Retro MTA extracted media was between 10 and 12 (Fig. 4B).
The effect of extracted medium on DPC osteogenic differentiation was investigated by evaluating the ALP activity of the cells and staining them for mineralized nodules. All three experimental groups in which cells survived (ProRoot MTA, Retro MTA, and Endocem Zr) exhibited reduced ALP activity compared to the negative control group, in which the cells were cultured only in growth medium; the lowest ALP activity was exhibited by the ProRoot MTA group (Fig. 5). However, more mineralized nodules were generated in the ProRoot MTA group than in all of the other experimental groups, and their number was only slightly lower than in the osteogenic medium (positive) control group. Both the Endocem Zr and Retro MTA groups exhibited little mineralized nodule generation. The degree of mineralized nodule generation was greater in all three experimental groups than in the negative control group, but less than in the osteogenic, positive control group (Fig. 6).
In vitro testing of the cell viability and differentiation potential of dental materials could lead to better treatment outcomes for vital pulp therapy procedures. However, few studies have focused on measuring these functional effects of the newly developed MTA-like materials on dental tissues. This study used human deciduous DPCs to evaluate the initial cell viability and differentiation responses to various MTA-like materials. Overall, there were no significant differences among the test materials with regard to both their cell viability and differentiation potentials. However, with indirect contact, Endocem Zr was associated with a slightly higher cell viability, possibly due to its lower alkalinity.
Endocem Zr is a zirconium-oxide-enriched, calcium-silicate-based cement [18]. A high zirconium content has been employed as a radiopacifier as a substitute of bismuth oxide [18]. Furthermore, the replacement of bismuth oxide with zirconium oxide may contribute to the fast setting properties and hence lower solubility of Endocem Zr [18], as observed in the SEM images produced in the present study. According to the manufacturer, Endocem Zr consists mainly of zirconia (59.49% wt), calcium (18.52% wt), and silicon (4.82% wt).
Retro MTA was developed as a bioceramic for root repair and vital pulp therapy. It consists of a hydraulic calcium-zirconia complex, and has a reduced setting time of 150 seconds. According to the manufacturer, Retro MTA consists of calcium carbonate (60-80% wt), silicon dioxide (5-15% wt), aluminum oxide (5-10% wt), and calcium-zirconia complex (20-30% wt), but it contains no heavy metals (http://www.biomta.com). One of the main differences between the more conventional products such as ProRoot MTA and Retro MTA is in their manufacturing processes: ProRoot MTA is produced through the application of various refining processes to Portland cement, whereas Retro MTA is synthesized by combining various chemical components.
As shown by the results of the present study, when used under direct-contact conditions, MTA exhibits a certain degree of cytotoxicity [10,19,20]. This cytotoxicity can be explained by the high pH created during setting, and which remains high for at least 8 weeks [11,21]. Previous studies have also reported this finding, although to a lesser degree, MTA has been shown to induce zones of inflammation and necrosis when it comes into direct contact with the pulp tissues [12,22]. Adjacent to this necrotic zone lies a newly formed calcific barrier [23]. Therefore, the bioactive influence of MTA on pulp tissue is thought to be mostly through indirect contact. Moreover, an in vitro study of MTA in indirect conditions better reflects the clinical conditions of vital pulp therapy, in which contact between the pulp cells and MTA occurs indirectly through the blood clot and physiologic fluids [24].
Studies of cell viability in indirect conditions have yielded controversial findings. Some have found an enhancement of cell viability with indirect contact with MTA [25-27], while others have produced contrary evidence, wherein both direct- and indirect-contact testing resulted in low cell viability [28], as observed in the present study. However, the diversity of experimental methods used in the different studies, and especially those used to prepare the test extracts, could be one of the factors influencing these differential results. ISO standard 10993-12:2007 calls for the extracts to be prepared so that the ratio of the surface area of the test material to the volume of the extracted medium is between 50 and 600 mm2/ml [29]. Even with strict adherence to this ISO standard, the variations in extract concentrations could produce different results.
Nonetheless, among the test materials, the relative cell count with indirect contact with Endocem Zr was considerably higher than for the other test materials. This finding may be attributable to the lower alkalinity of Endocem Zr compared to those of other MTA materials. The main chemical compound of MTA that causes and maintains this high pH is calcium hydroxide [21]; the relatively low alkalinity of Endocem Zr could therefore be explained by its low calcium content. Unlike ProRoot MTA and Retro MTA, in which calcium and silicon are the main constituents, Endocem Zr consists mainly of zirconia, with lower amounts of calcium and silicon [18]. The addition of high amounts of zirconium oxide has greatly reduced the amount of calcium released from Endocem Zr compared to other MTA materials.
The ideal repair process after pulpotomy requires a thick, firm, hard tissue barrier adjacent to the filling material, as well as a healthy pulp tissue below it. However, the exact mechanism by which MTA induces the formation of a hard-tissue bridge is only partly understood. It has been noted that the mechanism underlying the stimulation of repair by the deposition of mineralized tissue is dependent upon the pH and the ability to release various ions including calcium ion [24,30,31]. One may question the influence of such changes in pH is it an inevitable drawback or a component necessary for a favorable reaction toward pulpal repair? Despite the induction of an inflammatory reaction and coagulation necrosis of the living pulp tissues, it has been suggested that the formation of mineralized tissue is also stimulated by the high pH of MTA [31,32]. Many other studies support this suggestion by showing that the high alkalinity of the material plays a major role in the induction of a hard-tissue barrier by creating a favorable environment for cell division, matrix formation, and antimicrobial activity [21,33]. Further investigation is needed to discover the effect and the influence of the relatively low pH of Endocem Zr on the repair process in pulp tissues.
Cell differentiation potential appears to vary according to the concentration of MTA. It has been reported that MTA suppresses the differentiation of human DPCs when in indirect contact [34]. The present differentiation test results yielded similar results. However, these results conflict with many previous studies showing that ProRoot MTA exhibited enhanced differentiation potential [5,6,35]. Such a favorable bioactive property of ProRoot MTA has been explained by a reaction similar to that of calcium hydroxide [11,12,23]. The release of calcium ions from ProRoot MTA during its interaction with moist, phosphate-containing environments induces the formation of a hydroxycarbonate apatite layer on the surface of the cement [36]. However, the controversy regarding the variation in differentiation potentials between studies could be attributable to differences in experimental conditions, one of the most influential of which may be the concentration of test extracts used. For example, in the study of Guven et al. [37], who reported enhanced differentiation with MTA, the ratio of the surface area to volume used for preparation of the test extract was 125 mm2/ml, whereas that in the present study was 299 mm2/ml. Higher extract concentrations could result in a higher content of toxic materials and a higher pH, thus leading to less favorable test results.
A major shortcoming of in vitro tests such as those performed in this study is that they do not allow determination of the interaction between the material and the host tissue. Given that only a few clinical studies involving few subjects have investigated pulpotomy, all of which lack long-term evaluation, further in vivo/clinical studies should be encouraged, especially for the newly developed Endocem Zr and Retro MTA.
Despite their differences, ProRoot MTA, Retro MTA, and Endocem Zr were all associated with low cell viability and were unable to enhance the proliferation or differentiation of human DPCs in this study. However, the cell viability of human DPCs associated with Endocem Zr was greater than for the other test materials, probably due to its lower alkalinity. Further in vivo/clinical studies is required for unveiling whether such natures could contribute to a successful treatment outcome.
Fig. 1.
Changes in the surface topography of discs constructed using various MTA/MTA-like materials (ProRoot MTA, RetroMTA, Endocem Zr, and IRM) between before (0 day) and 3 days after cell culture, and cell adhesion to the test materials as analyzed using SEM. (A-D) Images were first obtained on day 0 without extraction. (E-H) Second images were obtained after culturing human deciduous DPCs on the test materials for 3 days (following 3 days of extraction with culture medium). Arrows indicate DPCs. Scale bars = 100 гОЫ in all photomicrographs.
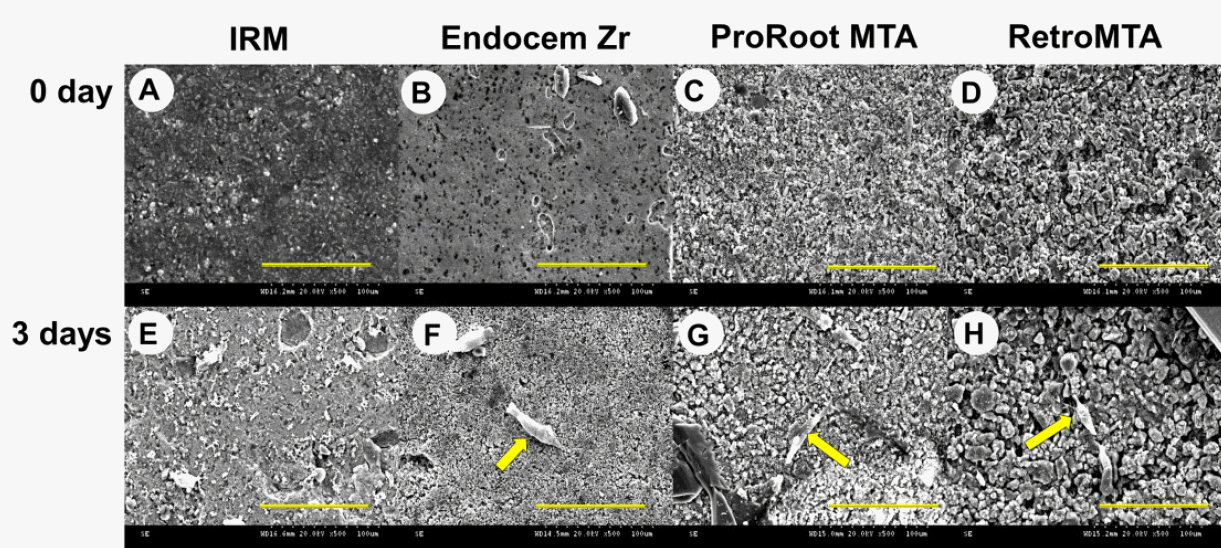
Fig. 2.
Relative cell count of DPCs in direct contact with various materials after 1 and 3 days of culture. The y-axis represents the percentage relative to the value for the control group (DPCs cultured on culture plates with growth medium), which was set at 100%. The data are presented as mean and SD values (n = 3). There was no statistical difference in DPC proliferation between any of the groups after 1 day (p > 0.01). However, significant differences (p < 0.01) were observed after 3 days between the Endocem Zr and ProRoot MTA groups (*), and the IRM and the ProRoot MTA and RetroMTA groups (ќ±).
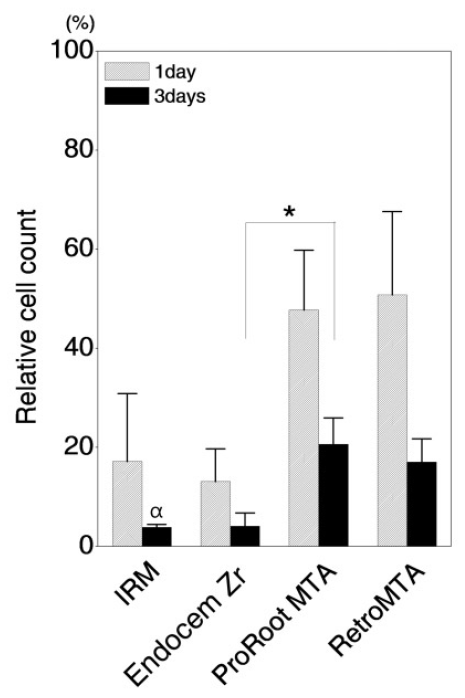
Fig. 3.
Relative cell count of DPCs after 3 days of indirect contact with media extracted from the various experimental materials diluted to concentrations of 1:1, 1:2, and 1:4. The y-axis represents the percentage relative to the value of the control group (DPCs cultured on culture plates with growth medium for 3 days), which was set at 100%. The data are presented as mean and SD values (n = 3). There were statistically significant differences (*) between the Endocem Zr and ProRoot MTA groups, and the Endocem Zr and RetroMTA groups at the 1:2 concentration, and between the Endocem Zr and RetroMTA groups at the 1:4 concentration. Statistically significant differences (ќ±) were also seen between the IRM group and the ProRoot MTA, RetroMTA, and Endocem Zr groups at all extracted medium concentrations (p < 0.01).
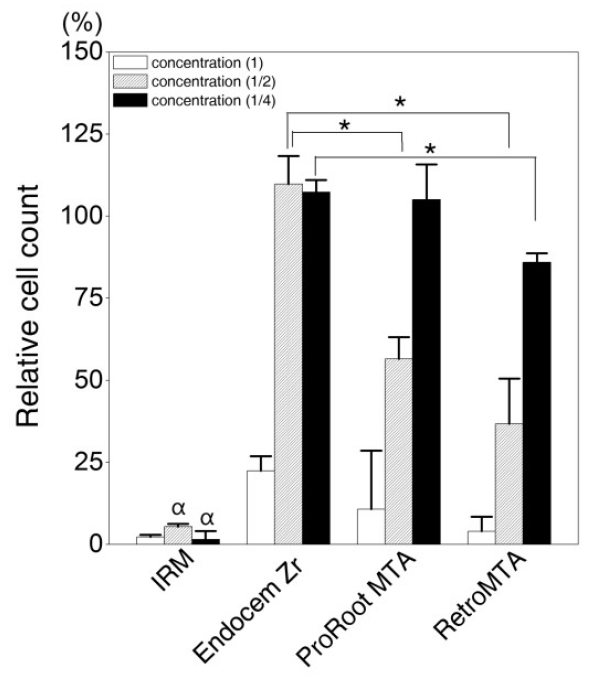
Fig. 4.
pH differences between the four experimental materials. (A) The pH of the experimental materials was visualized using Thymol blue and Alizarin Yellow R staining in test extracts at the 1:2 concentration. (B) The pH of the materials was also measured according to a dilution gradient. The data are presented as mean and SD values.

Fig. 5.
ALP staining and enzymatic activities of human DPCs in the three test-material extracts after 3 days of culture. (A) ALP staining images were obtained after 10 days of DPC exposure to the test materials in culture medium and compared to the control (i.e., not exposed to osteogenic factors; negative control) and osteogenic (i.e., osteogenic differentiation medium; positive control) groups. (B) ALP activities of DPCs in the test-material extracts. The y-axis indicates the relative activity of ALP, which is expressed as ALP (ng)/total protein (гОН). The data are presented as mean and SD values (n = 6). Statistically significant difference (*) was observed between the ProRoot MTA and osteogenic groups (p < 0.01).
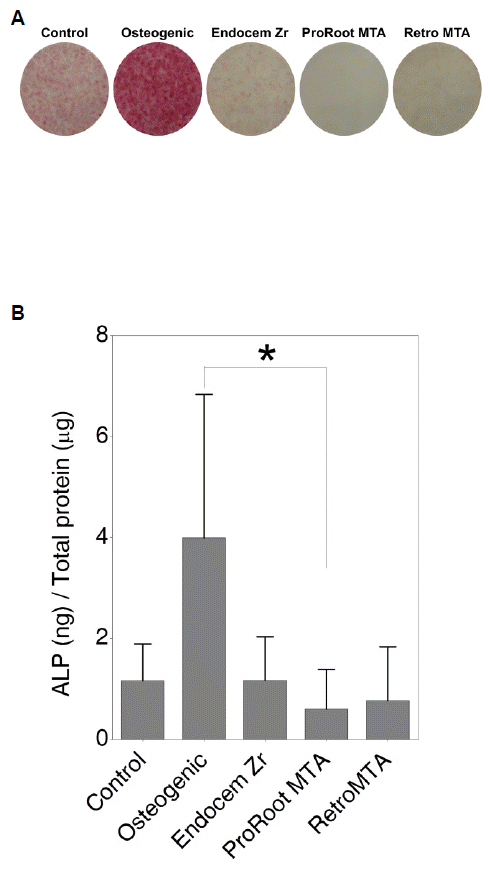
Fig. 6.
Degree of extracellular mineralization achieved by DPCs when cultured in media extracted from the experimental materials. (A) Images were obtained and compared to the control and osteogenic groups using Alizarin Red S staining. The stained mineralization nodules (lower) were observed using a light microscope. Scale bars=100 гОЫ. (B) The amount of stained nodules generated in each of the test materials in which cells survived was measured. The y-axis represents the optical density (OD) at 570 nm. The data are presented as mean and SD values (n=7). Statistically significant difference (ќ±) was seen between all test groups and the control group (p < 0.01). Statistically significant difference (*) was seen between the Endocem Zr and osteogenic groups (p < 0.01).

References
1. Roberts HW, Toth JM, Berzins DW, Charlton DG : Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater, 24:149-164, 2008.


2. Mente J, Leo M, Panagidis D, et al. : Treatment outcome of mineral trioxide aggregate in open apex teeth. J Endod, 39:20-26, 2013.


3. Godhi B, Sood PB, Sharma A : Effects of mineral trioxide aggregate and formocresol on vital pulp after pulpotomy of primary molars: An in vivo study. Contemp Clin Dent, 2:296-301, 2011.



4. Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S : Treatment outcomes of primary molars direct pulp capping after 20 months: a randomized controlled trial. 8:149-152, 2013.
5. Yasuda Y, Ogawa M, Arakawa T, et al. : The effect of mineral trioxide aggregate on the mineralization ability of rat dental pulp cells: an in vitro study. J Endod, 34:1057-1060, 2008.


6. Paranjpe A, Zhang H, Johnson JD : Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod, 36:1042-1047, 2010.


7. Ma J, Shen Y, Stojicic S, Haapasalo M : Biocompatibility of two novel root repair materials. J Endod, 37:793-798, 2011.


8. Lee BN, Son HJ, Noh HJ, et al. : Cytotoxicity of newly developed ortho MTA root-end filling materials. J Endod, 38:1627-1630, 2012.


9. Perinpanayagam H : Cellular response to mineral trioxide aggregate root-end filling materials. J Can Dent Assoc. 75:369-372, 2009.

10. Torabinejad M, Hong C, Pitt Ford T, Kettering J : Cytotoxicity of four root end filling materials. J Endod, 21:489-492, 1995.


11. Faraco IM Jr, Holland R : Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol, 17:163-166, 2001.


12. Holland R, de Souza V, Nery MJ, et al. : Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod, 25:161-166, 1999.


13. Zhou HM, Shen Y, Wang ZJ, et al. : In vitro cytotoxicity evaluation of a novel root repair material. J Endod. 39:478-483, 2013.


14. Guven EP, Yalvac ME, Sahin F, et al. : Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod, 37:650-656, 2011.


15. Zhao X, He W, Song Z, et al. : Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol Biol Rep, 39:215-220, 2012.


16. Kamon M, Zhao R, Sakamoto K : Green tea polyphenol (-)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol Int, 34:109-116, 2010.

17. Mah YJ, Song JS, Kim SO, et al. : The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch Oral Biol, 59:539-549, 2014.


18. Han L, Kodama S, Okiji T : Evaluation of calcium releasing and apatite forming abilities of fast setting calcium silicate based endodontic materials. 48:124-30, 2014.

19. Koulaouzidou EA, Economides N, Beltes P, et al. : In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA Angelus. J Oral Sci, 50:397-402, 2008.


20. Camilleri J, Montesin F, Di Silvio L, Pitt Ford T : The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J, 38:834-842, 2005.


22. Seux D, Couble ML, Hartmann DJ, et al. : Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch Oral Biol, 36:117-128, 1991.


23. Bakland LK, Andreasen JO : Will mineral trioxide aggregate replace calcium hydroxide in treating pulpal and periodontal healing complications subsequent to dental trauma. Dental Traumatol, 28:25-32, 2012.

24. Seo MS, Hwang KG, Lee J, et al. : The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod, 39:242-248, 2013.


25. Moghaddame-Jafari S, Mantellini MG, Botero TM, et al. : Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod, 31:387-391, 2005.


26. Damas BA, Wheater MA, Bringas JS, Hoen MM : Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J Endod, 37:372-375, 2011.


27. Hirschman WR, Wheater MA, Bringas JS, Hoen MM : Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod, 38:385-388, 2012.


28. Camilleri J, Montesin FE, Papaioannou S, et al. : Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J, 37:699-704, 2004.


29. Keiser K, Johnson CC, Tipton DA : Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod, 26:288-291, 2000.


30. Duarte MAH, Demarchi ACCdO, Yamashita JC, et al. : pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 95:345-347, 2003.


31. Okabe T, Sakamoto M, Takeuchi H, Matsushima K : Effects of pH on mineralization ability of human dental pulp cells. J Endod, 32:198-201, 2006.


32. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR : Physical and chemical properties of a new root-end filling material. J Endod, 21:349-353, 1995.


33. Accorinte MdLR, Holland R, Reis A, et al. : Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod, 34:1-6, 2008.


34. Paranjpe A, Smoot T, Zhang H, Johnson JD : Direct contact with mineral trioxide aggregate activates and differentiates human dental pulp cells. J Endod, 37:1691-1695, 2011.



35. Zhang S, Yang X, Fan M : BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J, 46:923-929, 2013.


- TOOLS
-
METRICS

-
- 3 Crossref
- 0 Scopus
- 4,837 View
- 86 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print