요오드화 나트륨을 사용한 유치 근관 충전재의 효과
Effectiveness of Sodium Iodide Root Canal Filling Pastes in Primary Teeth
Article information
Abstract
이 연구의 목적은 유치에 있어 요오드화 나트륨을 사용한 근관 충전재의 효과를 알아보는 것이다. 요오드화 나트륨 근관 충전재의 효과를 알아보기 위해 현재 유치에서 가장 많이 사용하고 있는 근관 충전재와 비교하여 물리적 특성 및 항균성을 평가하였다. 비교한 기존의 근관 충전재는 Vitapex®와 Metapex®이며, 평가한 물리적 특성은 유동성, 피막도, 방사선 불투과성이다. 또한 항균성은 Enterococcus faecalis 균주(ATCC 6538)를 대상으로 평가하였다. 유동성 및 피막도는 기존의 근관 충전재와 요오드화 나트륨 근관충전재에서 통계적으로 유의미한 차이를 보이지 않았다(p > 0.05). 요오드화 나트륨 근관충전재는 방사선 불투과성이 기존의 충전재에 비해 더 낮은 결과값을 보였으며 이는 통계적으로 유의미한 차이를 보였다(p < 0.05). 요오드화 나트륨 근관충전재가 기존 충전재보다 더 높은 항균성을 보였으며 이 또한 통계적으로 유의미한 상관관계를 나타냈다(p < 0.05). 요오드화 나트륨 근관 충전재는 기존의 상품화된 근관 충전재와 효과를 비교하였을 때 유동성이나 피막성에 대해 떨어지지 않는 성능을 보였으며, 항균성에 대해서는 더 우월한 결과를 보여 기존 충전재의 대안제로 고려해 볼 수 있을 것으로 사료된다.
Trans Abstract
Objectives
This study aimed to compare the physical properties and antibacterial effectiveness of iodoform based root filling pastes, Vitapex® and Metapex®, with sodium iodide root filling paste (NaI paste) for primary teeth.
Materials and Methods
The physical properties (flowability, film thickness, radiopacity) of the pastes were evaluated according to ISO 6876:2012. The antibacterial activity against Enterococcus faecalis strain (ATCC 6538) was evaluated using a direct contact test.
Results
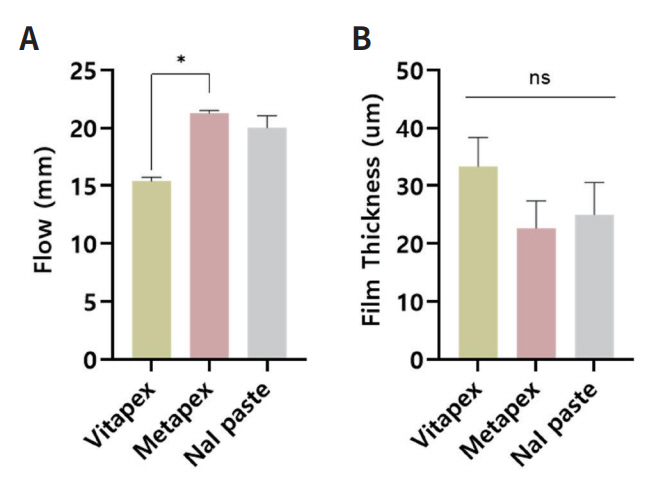
There was no significant statistical difference (p > 0.05) observed in the flow and film thickness of NaI paste when compared to the currently available root canal filling materials. The average flow capacities for Vitapex®, Metapex®, and NaI paste were 15.40 mm, 21.25 mm, and 20.01 mm, respectively. The average film thickness for Vitapex®, Metapex®, and NaI paste were 33.3 μl, 22.6 μl, and 25.0 μl, respectively. However, NaI paste showed lower radiopacity than the existing materials, and this difference was statistically significant (p < 0.05) NaI paste demonstrated higher antimicrobial activity than the available materials, and this difference was also statistically significant (p < 0.05).
Conclusion
Compared to the existing commercialized root canal filling materials, NaI paste exhibited similar performance in terms of flow and film thickness, and superior antimicrobial activity against E. faecalis . Hence, NaI paste could be a promising root filling material for primary teeth and may be a potential alternative to existing materials.
Introduction
For successful pulpectomy treatment in the primary tooth, it is important to seal all canals seamlessly using filling materials after the removal of microorganisms that were present in the root canal [1]. The ideal root-filling material should be easy to insert and remove, radiopaque for viewing on radiographs, and antiseptic in the root canal [2]. Complete filling of endodontic material is crucial for the success of endodontic treatment of deciduous teeth [3]. The optimum root filling material, according to Grossman, should be bacteriostatic [4]. Toxicity and antibacterial effectiveness are essential requirements to maintain the optimum root filling condition [5-7]. It is necessary to maintain a resorption rate equivalent to that of physiologic resorption [8]. The timely exfoliation of primary teeth, in conjunction with the emergence of their successors, is essential to prevent various complications, such as impaction or proximal tooth displacement.
Various local factors, such as inflammation from decay, pulp necrosis, and pulp treatment, affect root resorption [9,10]. Moskovitz et al. discovered that root filling materials in primary molars could expedite root resorption due to tissue irritation near the root apex. The study suggested that the iodoform-containing filling material could cause dental follicle irritation, leading to accelerated root resorption [11]. Iodoform based root filling material such as Vitapex® (Neo International, Federal Way, WA, USA), a premix of calcium hydroxide and iodoform, is regarded as the gold standard in filling materials for primary teeth, with high success rates [12]. A prior research indicated that Sodium Iodide (NaI) could be used as a substitute for iodoform to reduce root resorption [13]. This conclusion was drawn based on the observation of a lower level of osteoclast differentiation in RAW 264.7 cells when exposed to NaI paste compared to iodoform based paste, as osteoclasts are responsible for resorption activity [14]. Further studies are necessary to explore the correlation between NaI paste and the rate of root resorption.
NaI is widely utilized as a diagnostic tool for thyroid gland evaluation in medical settings [15]. Additionally, NaI is sometimes used to treat iodine deficiency, hyperthyroidism, and as a supplement in total parenteral nutrition [16]. Despite the extensive usage of NaI in various medical applications, research on its effectiveness in dentistry and the antibacterial potency of NaI as a root filling material is lacking.
Several iodoform-based pastes had been used in prior studies. Iodoform based pastes mixed with calcium hydroxide exist under the commercial brands currently known as Metapex® (Meta Biomed, Cheongju, Korea) and Vitapex® [17]. Although Vitapex® and Metapex® are similar in their compositions, practically all studies have solely examined Vitapex® [18-20]. Therefore, the purpose of this study was to compare the physical characteristics and antibacterial efficacy against Enterococcus faecalis of iodoform-based paste (Vitapex® and Metapex®) and NaI paste. We are not aware of any research that has examined the antibacterial potency of sodium iodide utilized as a root-filling material. Our null hypothesis was that NaI paste would demonstrate lower physical properties and less antibacterial efficacy than Vitapex® and Metapex®.
Materials and Methods
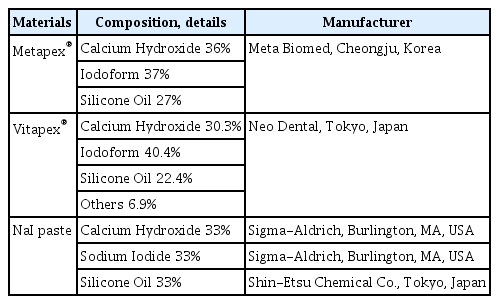
1. Material preparation
This research compared iodoform-based pastes, Vitapex ® and Metapex®, with sodium iodide (NaI) paste. NaI paste was created by combining sodium iodide (Sigma-Aldrich, Burlington, MA, USA), calcium hydroxide (Sigma-Aldrich), and silicone oil (Shin-Etsu Chemical Co., Tokyo, Japan) in a 1 : 1 : 1 proportion (Table 1). The mixture was not suitable as a root-filling material when the proportion was altered to be similar to Vitapex® or Metapex®. The NaI pastes were mixed by the same operator.
2. Methods
1) Physical properties
The present study evaluated the flow, film thickness, radiopacity of Vitapex®, Metapex®, and NaI paste using ISO 6876:2012 protocols (International Organization for Standardization ISO 6876:2012 - Dental root sealing materials). The purpose of this protocol is to ensure that the root filling material is within acceptable limits, which is essential for its clinical performance. The ISO standard values for flow, film thickness, and radiopacity are established at 17 mm, 50 μm, and 3 mm Al respectively. All tests were conducted in triplicate at a relative humidity of 23 ± 2℃, unless stated otherwise, and mean values were used for comparisons.
(1) Flow
A 0.05 mL mixture was placed on a 40.0 ± 0.1 mm in diameter and 5.0 ± 0.1 mm in glass plate using a disposable syringe. Three minutes after mixing began, a second glass plate of the same size and height was added and a 100 g mass was placed on top for 7 minutes. The maximum and minimum diameters were measured with a digital caliper (Mitutoyo, Mitutoyo Corp., Kawasaki, Japan) after removing the 100 g mass.
(2) Film Thickness
Two glass plates (15.0 ± 0.1 mm width, 5.0 ± 0.1 mm height) were measured for their combined thickness using a micrometer (Mitutoyo, Mitutoyo Corp.). Then, 0.01 mL of the combined materials were pipetted onto one plate, and the other plate was placed on top. After 3 minutes, a load of 150 N was applied vertically on top of the glass plate. After 10 minutes, the thickness of the two plates with the specimens was measured again using a micrometer. The difference between the initial and final thickness was calculated.
(3) Radiopacity
To assess radiopacity, each combined material was placed in a mold (10.0 ± 0.1 mm diameter, 1.0 ± 0.1 mm height) and imaged alongside an aluminum step wedge using a digital sensor (Kodak Insight, Rochester, NY, USA). Images were captured under specific conditions (65 ± 5, kV 7mA, 0.3 sec with 300 ± 100 mm distance from film to x-ray) and analyzed using the Grayscale program (ImageJ version 1.53a, National Institutes of Health, Bethesda, MD, USA) to determine the densities of the different pastes and aluminum step wedge thickness [21]. The optical densities of the specimens were then converted to aluminum wedge thickness using the formula by Duarte et al. [22].
2) Optical microscope image
To visualize the surface of each paste, 0.01 mL of sealer was examined under a 25× optical microscope (S39A, Microscopes Instrument) on a glass plate.
3) Antibacterial test
The bacterial strain, Enterococcus faecalis (E. faecalis , ATCC 6538, USA) was used for all tests. Cultures were grown overnight in Brain Heart Infusion broth (BHI, Difco, BD Diagnostic Systems, Sparks, MD, USA) at 37°C in a shaking incubator. A 0.3 g specimen was coated in each well of a culture plate (n = 5) using a cavity liner applicator, and 3 mL of 3 × 106 CFU/mL E. faecalis was added on top. The positive control group did not use any paste. The well was placed in a 37°C 5% CO2 incubator for an hour and then 300 μL PrestoBlueTM (Molecular probes, Eugene, OR, USA) was added as a cell viability indicator. Every 30 minutes, 100 μL were collected in a 96-well plate and the absorbance at 570 nm and 600 nm was measured using a microplate reader (BioTek, Winooski, VT, USA). Cell viability was determined by subtracting the values from OD570 and OD600, which show the linear relationship between CFU and Prestoblue absorbance readings [23].
4) Statistical Analysis
Statistical analysis was performed using the Graph-Pad Prism 8 software (GraphPad software, San Diego, CA, USA). To assess for significant differences between groups, Kruskal-Wallis test and Dunnett’s multiple comparison tests were used.
Results
1. Physical properties
1) Flow
ISO 6876:2012 requires a minimum flow of 17 mm. In the flow test (Fig. 1A), all groups met this requirement, except for Vitapex®. Vitapex® and Metapex® showed a significant difference (p < 0.05), while NaI paste and Metapex® showed no statistical difference (p > 0.05).
2) Film thickness
All the tested groups had a film thickness below 50 μm which meets the standard requirement of ISO 6876:2012 (Fig. 1B). There was no significant difference observed among the groups (p > 0.05).
3) Radiopacity
A root canal sealer must be radiopaque enough to be distinguishable from surrounding anatomical structures [24,25]. The minimum requirement of ISO 6876:2012 standards is that sealers should be above 3 mm of aluminum thickness. In our study, all materials showed radiopacity above the minimum requirement (Fig. 2A). Vitapex ® and NaI paste had significant differences, and so did Metapex® and NaI (p < 0.05). However, there was no significant difference in the radiopacity levels between Vitapex® and Metapex® (p > 0.05). Please refer to Fig. 2B for the radiopacity images.

(A) Radiopacity of Vitapex®, Metapex®, NaI paste groups. (B) Radiographic image of the radiopacities of Vitapex® (a), Metapex® (b), NaI paste (c) groups and their equivalence to those of the aluminum step wedge. The data are presented as mean and standard deviation values (n = 3).
* letters mean a statistically significant difference among groups.
2. Optical microscope image
Under microscopic examination, Vitapex® appeared to be sticky and amorphous in form. In contrast, Metapex® and NaI paste exhibited a smooth and solid formation with a glossy surface. Additionally, Vitapex® and Metapex® displayed a yellow color, while NaI paste presented a greyish-white hue (Fig. 3). These observations suggest differences in the physical characteristics and composition of these materials.
3. Antibacterial test
The results indicate that Metapex® and NaI paste have a significant difference compared to the control group, as shown in Fig. 4. However, although Vitapex® demonstrated lower bacterial viability compared to the control group, no significant difference was observed. Notably, NaI paste demonstrated the lowest bacterial viability value among the three materials. These findings suggest that NaI paste may have superior antibacterial properties compared to Vitapex® and Metapex®.
Discussion
Dentists commonly utilize root filling materials that contain iodine to avert infection. These materials have a powerful response that results in the precipitation of proteins and the oxidation of vital enzymes [26]. Pastes containing iodoform have consistently been recommended as antiseptics over the years due to iodine release when they come into contact with secretion or endodontic infections [26]. Iodoform, which has a molecular weight of 393.73 g/mol, is a powder consisting of lemon-yellow colored hexagonal crystals. It emits a strong and long-lasting odor and is only slightly soluble in water [26]. As a substitute for iodoform, sodium iodide was introduced as a possible option for root canal filling in primary teeth. Sodium iodide is an alternative source of iodine that is similar to iodoform. However, it has a different molecular weight of 149.89 g/mol and is watersoluble. In a previous study [13], the physicochemical properties of sodium iodide paste were compared to those of iodoform paste, which is blended in a ratio of 1 : 1 : 1 with calcium hydroxide and silicone oil. As such, this current investigation endeavors to assess the potential applicability of sodium iodide paste by examining its effectiveness in comparison to commercially available iodoform paste. Therefore, we conducted a comparison between the commercial iodoform root filling materials, Vitapex® and Metapex®, and evaluated NaI paste as a possible root filling material for use in pediatric dentistry.
According to this study, NaI paste was found to be more favorable than Vitapex® and Metapex® in achieving high flow with a thin layer of film. Vitapex® is regarded as the gold standard for root filling materials for primary teeth, even though it did not exceed the ISO standards test in this study. We think that the variations in the percentages of liquid components in the pastes may have had an impact on the outcomes in terms of flow and film thickness. Vitapex® contains the lowest percentage of silicone oil (22%) compared to other pastes, Metapex® (27%) and NaI (33%). Also, the outcome may have been impacted by the increased quantity of calcium hydroxide, which is often used to reduce flow in several endodontic filling materials [27,28]. Our findings reveal that the differences in water solubility among these pastes did not elicit any significant impact on their physical properties. While there was a noticeable difference between NaI paste and Vitapex® in flow, there was no observable distinction between NaI paste and Metapex®. The superior flow capacity exhibited by NaI paste, in comparison to Vitapex®, may potentially yield increased filling efficacy with greater penetration into dentinal tubules [12,29]. Nonetheless, the utilization of highly flowable materials in root canal treatment carries the potential risk of extrusion beyond the apex, culminating in adverse outcomes such as foreign body reactions, pain symptoms, and infective periapical periodontitis [30,31]. Hence, it is imperative that endodontic materials possess biocompatibility, a characteristic typically evaluated through cytotoxic assays [32]. According to prior investigations, pastes containing iodoform exhibited a noteworthy survival rate in both human osteosarcoma cell lines and animal models [33,34]. Furthermore, it has been observed that NaI pastes display high cell viability on osteoclast precursor cells [13]. This observation suggests that both iodoform-based pastes and NaI pastes may demonstrate biocompatibility when extruded over the apex; however, additional clinical investigations are required to validate these results.
For endodontic filling materials, radiopacity is an essential factor for determining whether the substance has filled the root canal completely or not. Our findings demonstrated that every tested paste exceeded the required 3 mm Al radiopacity levels and complied with ISO standards. The atomic number, density, and size of the chemicals in the root canal paste have a significant impact on how x-rays interact with matter at the atomic level [35,36]. Since sodium iodide (molecular weight 149.89 g/mol) has a lower atomic number than that of iodoform (393.732 g/mol), NaI paste showed the lowest radiopacity when compared to Vitapex® and Metapex®, which was consistent with a previous study [13]. However, there was no statistically significant difference among the groups.
Optical imaging was used to examine the surface and texture of various root filling materials. It was observed that Vitapex® exhibited a sticky quality, whereas Metapex® and NaI paste were more evenly distributed. This suggests that Vitapex® may retain its shape and adhere to the canal wall without spreading further into the dentinal tubules when applied in the root canal. In contrast, Metapex® and NaI paste may have better penetration ability into complex accessory canals. However, it is important to note that optical imaging has limitations in evaluating interfacial gaps and voids unless root canals are partitioned and assessed. Therefore, further investigation is necessary to visualize the distribution of pastes in the root canal system.
Studies have shown the Enterococcus faecalis (E. faecalis) is commonly found in cases where post-treatment disease has occurred [37,38]. Typically, a primary root canal infection involves a microbiota primarily composed of gram-negative anaerobic bacteria. However, in cases of post-treatment disease, the microbiota is predominantly composed of gram-positive organisms, particularly E. faecalis [39]. This species has demonstrated high resistance to the medicaments commonly used during treatment and is known to withstand the antibacterial effects of calcium hydroxide dressings [40].
The antibacterial effectiveness of Vitapex® against E. faecalis shows different results based on the method of experiments. The agar diffusion method has been widely used to test the antimicrobial activity of root canal sealers [41,42]. The size of the microbial inhibition zone depends on the solubility test substance in the agar diffusion method, in which water-insoluble pastes may not express their full potential [43]. This was in accordance with previous studies where the agar diffusion test showed slight to no results for iodoform-based pastes [1,6,44]. The direct exposure method is correlated to substance effectiveness and its direct contact with microorganisms. This method is independent of other variables and is practical to use in the laboratory, where Vitapex® showed antibacterial results [6,37]. Therefore, for this study, the direct contact method was used.
The antibacterial properties of Vitapex® are largely attributed to the presence of calcium hydroxide and iodoform [18]. Calcium hydroxide dissolves in aqueous fluids, releasing calcium and hydroxyl ions, which gives it a highly alkaline pH that is bactericidal [45]. The mechanism of action of iodoform is not fully understood, but it is thought to release iodine, which oxidizes bacterial proteins, resulting in bacteriostatic action [46,47]. While the mechanism of sodium iodide remains unclear, the present study indicates that it has higher antibacterial activity than iodoform-based materials, Vitapex® and Metapex®, as demonstrated by its lower bacterial viability. The calcium hydroxide and iodoform in Vitapex® and Metapex® contribute to their antibacterial activity, as expected. However, NaI paste exhibited the highest antibacterial activity, suggesting that sodium iodide is a more effective bactericidal agent than iodoform. Although high antimicrobial activity can sometimes be associated with high cell toxicity, a study by Choi et al., found that NaI paste did not induce cell cytotoxicity, suggesting that it may be effective against bacteria within dentinal tubules without being toxic [13]. Nevertheless, this study was conducted in vitro over a short period of time, and further research is needed to validate these findings in vivo.
Our null hypothesis was rejected. NaI paste met all of the criteria outlined in ISO standards for physical properties and showed satisfactory results. Additionally, NaI paste demonstrated superior antimicrobial activity against E. faecalis when compared to Vitapex® and Metapex®, making it a promising option for use as a root filling material in primary teeth.
Conclusion
Compared to the existing commercialized root canal filling materials, sodium iodide exhibited similar performance in terms of flow and film thickness, and superior antimicrobial activity against E. faecalis . Based on the findings, it can be inferred that NaI paste has the potential to serve as a viable substitute for current root canal filling materials in primary teeth, which may hold promise as a material of choice in the field of pediatric dentistry.
Notes
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Funding information
This research was funded by the Department of Dentistry (Pediatric dentistry) supported through the Research-Focused Department Promotion Project as a part of the University Innovation Support Program for Dankook University in 2021 (2020R1G1A1009155).
Acknowledgements
This research was funded by the Department of Dentistry (Pediatric dentistry) supported through the Research-Focused Department Promotion Project as a part of the University Innovation Support Program for Dankook University in 2021 (2020R1G1A1009155).