네 가지 규산 칼슘계 시멘트의 경화시간, 용해도, 압축강도 평가
Evaluation of Setting Time, Solubility, and Compressive Strength of Four Calcium Silicate-Based Cements
Article information
Abstract
이 연구의 목적은 4가지 규산 칼슘계 시멘트를 대상으로 물리적 특성을 비교하고 평가하는 것이다. 2종의 분말-용액 혼합형 재료인 RetroMTA® [RTMX], Endocem® MTA Zr [EZMX] 그리고 2종의 기혼합형 재료인 Well-Root™PT [WRPR], Endocem MTA® premixed [ECPR]를 사용하여 경화시간, 용해도 및 압축강도를 비교하였다. 가장 짧은 경화 시간은 EZMX (123.33 ± 5.77초)에서 관찰되었으며, RTMX (146.67 ± 5.77초), ECPR (260.00 ± 17.32초) 및 WRPR (460.00 ± 17.32초) 순으로 증가하였다. 가장 높은 용해도는 WRPR (9.01 ± 0.55%)에서 관찰되었으며, RTMX (2.17 ± 0.07%), EZMX (0.55 ± 0.03%) 및 ECPR (0.17 ± 0.03%) 순으로 감소하였다. 또한 압축강도는 ECPR (76.67 ± 25.67 Mpa)에서 가장 높게 나타났고, WRPR (38.39 ± 7.25 Mpa), RTMX (35.07 ± 5.34 Mpa), EZMX (4.07 ± 0.60 Mpa) 순으로 감소하였다. 결론적으로 기혼합형 규산 칼슘계 시멘트들은 분말-용액 혼합형에 비해 긴 경화 시간을 나타내었다. 용해도 실험 결과 가장 낮은 용해도를 보인 ECPR과 가장 높은 용해도를 보인 WRPR에서 통계적 차이가 관찰되었다(p < 0.0083). 압축강도 실험결과 가장 낮은 압축 강도를 보인 EZMX와 가장 높은 압축 강도를 보인 ECPR에서 통계적 차이가 관찰되었다(p < 0.0083). ECPR은 분말-용액 혼합형에 비해 긴 경화 시간을 나타내지만, 미리 혼합되어 있어 혼합 시간이 필요하지 않고 용해도와 압축 강도가 개선되었으므로 임상 사용 시 선택될 수 있는 유망한 재료이다.
Trans Abstract
This study aimed to compare the physical properties of 4 kinds of calcium silicatebased cements (CSCs): 2 kinds of powder-liquid mix type (RetroMTA® [RTMX] and Endocem® MTA Zr [EZMX]) and 2 kinds of premixed type (Well-Root™PT [WRPR] and Endocem® MTA premixed [ECPR]) CSCs, respectively. Further, we assessed the setting times, solubility values, and compressive strengths of the cements. The shortest setting time was observed for EZMX (123.33 ± 5.77 seconds), followed by RTMX (146.67 ± 5.77 seconds), ECPR (260.00 ± 17.32 seconds), and WRPR (460.00 ± 17.32 seconds), respectively. The highest solubility was observed for WRPR (9.01 ± 0.55%), followed by RTMX (2.17 ± 0.07%), EZMX (0.55 ± 0.03%), and ECPR (0.17 ± 0.03%). Furthermore, the highest compressive strength was observed for ECPR (76.67 ± 25.67 Mpa), followed by WRPR (38.39 ± 7.25 Mpa), RTMX (35.07 ± 5.34 Mpa), and EZMX (4.07 ± 0.60 Mpa). In conclusion, the premixed type CSCs (WRPR and ECPR) exhibited longer setting times compared to the powder-liquid mix type CSCs (EZMX and RTMX). The solubility test showed that ECPR had the lowest solubility while WRPR had the highest solubility, with a statistically significant difference between them (p < 0.0083). Additionally, the compressive strength test showed that ECPR had the highest compressive strength, while EZMX had the lowest compressive strength, also with a statistically significant difference between them (p < 0.0083). ECPR is a promising material as it is premixed, eliminating the need for mixing time, and it has also demonstrated improved solubility and compressive strength, making it a potentially favorable option for clinical use.
Introduction
Vital pulp therapy (VPT) is a conservative treatment approach that aims to maintain the vitality and function of the dental pulp in teeth with carious or traumatic exposure [1]. It is considered an effective treatment option for primary teeth to manage pulp exposures and avoid the need for more invasive treatments [2]. Furthermore, for primary teeth, the two main types of VPTs are indirect pulp capping and pulpotomy [3]. Notably, indirect pulp capping is used to protect the pulp tissue and induce the formation of a layer of reparative dentin, which acts as a barrier against further bacterial invasion [4]. This procedure involves the removal of only the caries-affected dentin and the placement of a biocompatible material (typically calcium hydroxide) over the remaining dentin, followed by the restoration of the lesion [3,5-7].
However, a previous long-term clinical study reported that the use of calcium hydroxide-based pulp capping agents may lead to increased treatment failure rates over time [6,8,9]. Mineral Trioxide Aggregate (MTA) has been proposed as an alternative material to calcium hydroxide for pulp capping owing to its superior sealing ability and biocompatibility [10].
However, early forms of MTA had several disadvantages, including tooth discoloration, high cost, long setting time, and the need for a revisit for final restoration [11]. Thus, improved MTA-like calcium silicate-based cements (CSCs), such as RetroMTA® [RTMX] (BioMTA, Seoul, Korea), Endocem Zr® [EZMX] (Maruchi, Wonju, Korea), Well-Root™ PT [WRPR] (Vericom, Chuncheon, Korea), and Endocem® MTA premixed [ECPR] (Maruchi), were developed subsequently [12]. Premixed CSCs, such as WRPR and ECPR, are a practical choice for dentists because they can be easily injected in precise amounts, thereby reducing waste and increasing efficiency [13,14]. However, given that these materials are relatively new to the market, the information about them remains limited, and only a few studies have investigated their physical properties.
Previous studies have suggested that modifying the various components of CSCs by adding elements to alleviate their main drawbacks can improve setting time. However, this may also impact other important characteristics, such as compressive strength and solubility [15-18]. The setting time is a crucial factor in endodontic treatment procedures because prolonged setting time can result in material washout [19,20]. In addition, materials with high solubility are easy to wash out, can aggravate pulp inflammation caused by bacterial infection, and can affect pulp response [21]. Compressive strength is essential to withstand high masticatory forces. This property is highly requested, especially in the molar region [22]. Therefore, the present study aimed to compare the setting time, solubility, and compressive strength of four different CSCs.
Materials and Methods
1. Sample preparation
All materials were evaluated after setting was performed according to the manufacturer’s instructions. RTMX was mixed with the supplied liquid at a W/P ratio of 3 drops per 0.3 g, and EZMX was mixed with sterile distilled water (DW) at a W/P ratio of 0.14 cc per 300 mg. For the premixed types of cements (i.e., WRPR and ECPR), as there were no instructions provided by the manufacturer regarding the amount of liquid to be used, the size of the samples was maintained the same as that for RTMX or EZMX, and the experiments were conducted using the same amount of sterile distilled water as that for EZMX (0.14 cc). The composition of each material is described in Table 1.
Since the requirements for calcium silicate-based materials have not yet been clearly defined, the setting time and solubility tests were performed according to the root canal sealer method proposed by the International Organization for Standardization (ISO) 6876:2012 [23]. Additionally, the compressive strength test was performed according to the water-based cement method proposed by ISO 9917-1:2007 [24].
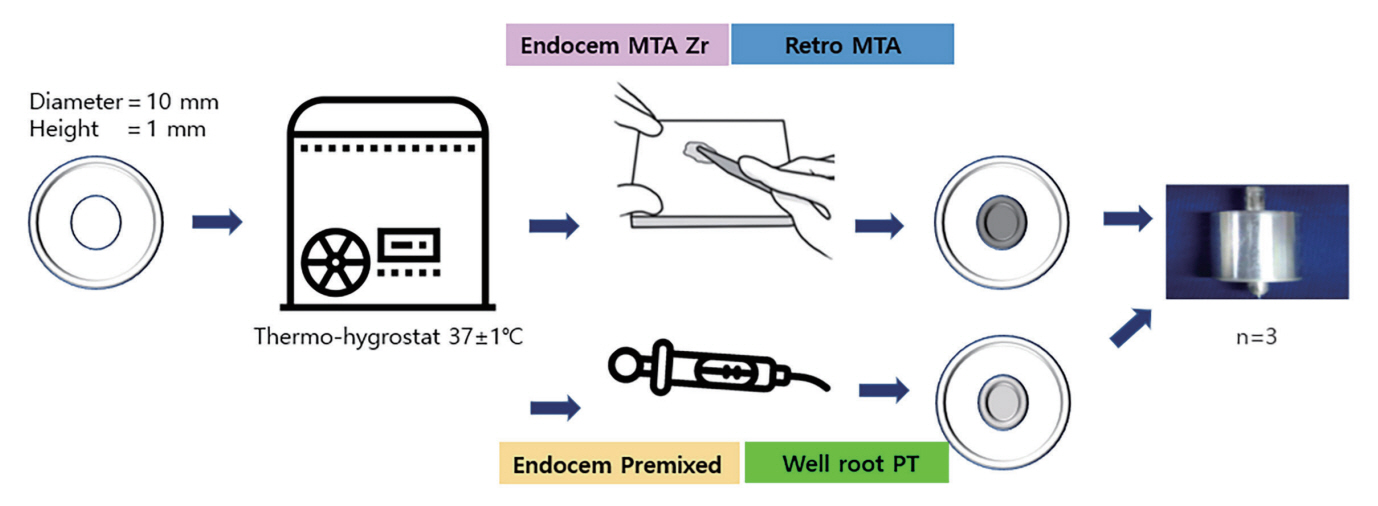
2. Setting time
The setting time experiment was conducted according to ISO 6876. For materials that do require moisture for setting, a gypsum mold (complying with Type 2 of ISO 6873 and consisting of a cavity [diameter, 10 mm; height, 1 mm]) was used to measure the setting times of the materials under investigation. First, the mold was stored at 37°C for 24 hours. Subsequently, the cavity in the mold was filled with CSCs and placed in a cabinet. A Gilmoretype indenter was lowered vertically onto the surface of the samples 30 seconds before the setting time specified by the manufacturer. Further, indentations were repeated at 10-second intervals. The final setting time was established as the time from the start of mixing to the time when no indentation was detected on the surface of the specimen. To minimize errors in the mixing process, a mixing pad was stored in a thermo-hygrostat at 37°C, and the mixing time was limited to ≤ 60 seconds (Fig. 1).
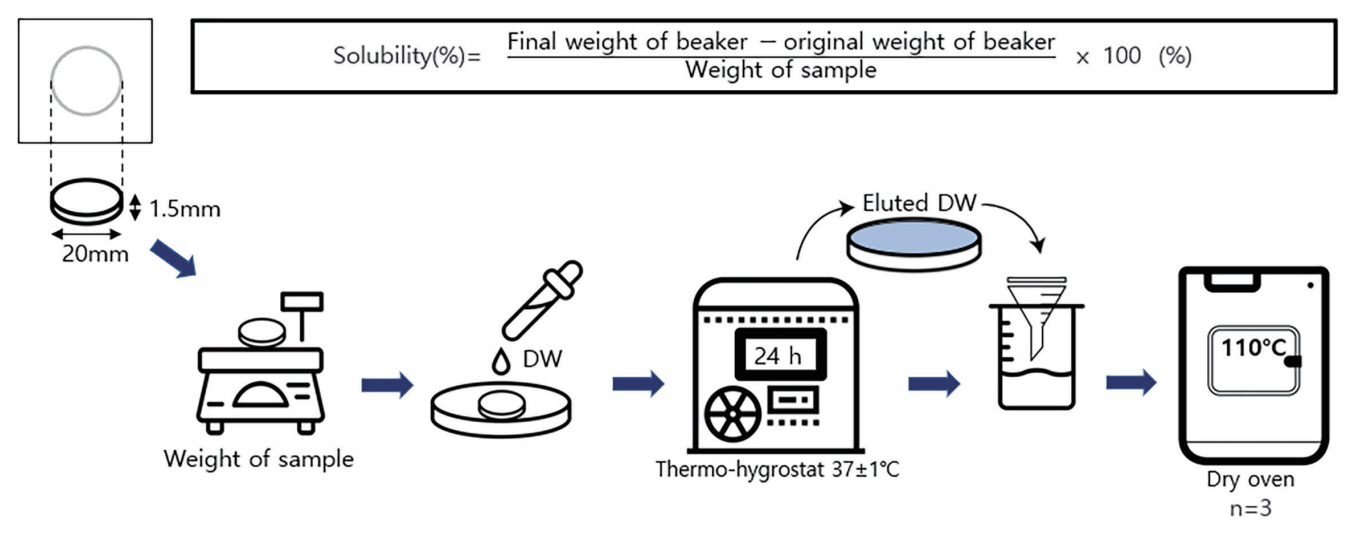
3. Solubility
The solubility experiment was conducted according to ISO 6876. Three samples of each material were placed on a Teflon mold (inner diameter, 20.0 mm; height, 1.5 mm), which was stored for 24 hours in thermo-hygrostat at 37°C. After removing the specimen from the mold, its net weight was evaluated three times. The average of the results was recorded using a fine scale to the nearest 0.001 g. One specimen was immersed in a shallow dish containing 50 mL of DW. After 24 hours, the specimen was removed, and the eluted DW was filtered through a funnel and poured into the beaker. Further, DW was dried in an oven at 110°C; subsequently, the solubility was measured by dividing the difference between the weight of the specimen and the original weight of the beaker by the initial weight of the specimen and then multiplying the value by 100 (Fig. 2).
4. Compressive strength
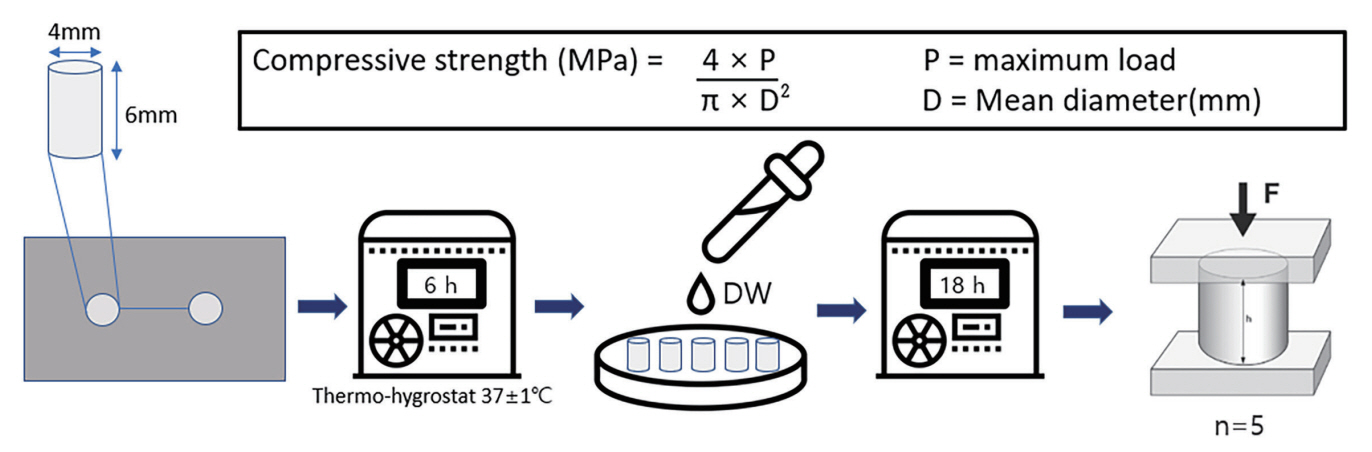
1) Specimen preparation
The compressive strength experiment was conducted according to ISO 9917-1. All materials were placed in split stainless-steel molds (internal dimensions: height, 6.0 ± 0.1 mm; inner diameter, 4.0 ± 0.1 mm) and transferred to the thermo-hygrostat, which was maintained at 37°C for 6 hours. Subsequently, the specimens were immersed in DW. After setting, the specimens were removed and visually inspected for air voids or broken edges. Subsequently, all defective specimens were discarded. A universal testing machine (Instron 3366, Instron Corp., High Wycombe, UK) was used to measure the compressive strength. The specimen was placed such that it enabled the application of a load on its long axis; the load was applied at a speed of 1.0 mm/min until a fracture occurred. The maximum load value required to cause fracture in the specimen was recorded (Fig. 3).
5. Statistical analysis
All data from the repeated tests were presented as mean ± standard deviation and analyzed using the Kruskal-Wallis test, followed by the Mann-Whitney U test with Bonferroni correction, using the Statistical Package for Social Sciences software version 21.0 (SPSS Inc., Chicago, IL, USA). A p-value of < 0.0083 was considered statistically significant.
Results
1. Setting time
The setting times (in seconds) of the cements are summarized in Table 2 and Fig. 4. EZMX had the shortest setting time (123.33 ± 5.77 seconds), followed by RTMX (146.67 ± 5.77 seconds), ECPR (260.00 ± 17.32 seconds), and WRPR (460.00 ± 17.32 seconds). ECPR and WRPR, which are premixed type CSCs, had longer setting times.

Schematic representation of the setting time results (n = 3).
p-value obtained from the Kruskal-Wallis test and the Mann-Whitney U test with Bonferroni correction.
n = number of measurements. The data are shown as mean ± SD values of 3 samples.
The lowercase letters indicate statistically significant differences (p < 0.0083).
RTMX: RetroMTA®; EZMX: Endocem® MTA Zr; WRPR: Well-RootTM PT; ECPR: Endocem® MTA premixed.
2. Solubility
The solubility values (in%) of the cements are summarized in Table 2 and Fig. 5. WRPR had the highest solubility value (9.01 ± 0.55%), followed by RTMX (2.17 ± 0.07%), EZMX (0.55 ± 0.03%), and ECPR (0.17 ± 0.03%). Significant differences were observed in terms of setting times among EZMX, ECPR, and WRPR and among RTMX, ECPR, and WRPR (p < 0.0083). In contrast, EZMX and RTMX showed no significant difference regarding their setting time (p > 0.0083).
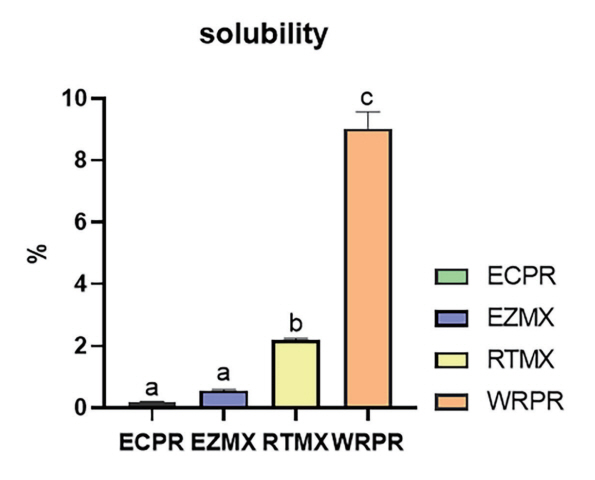
Schematic representation of the solubility results (n = 3).
p-value obtained from the Kruskal-Wallis test and the Mann-Whitney U test with Bonferroni correction.
n = number of measurements. The data are shown as mean ± SD values of 3 samples.
The lowercase letters indicate statistically significant differences (p < 0.0083).
RTMX: RetroMTA®; EZMX: Endocem® MTA Zr; WRPR: Well-RootTM PT; ECPR: Endocem® MTA premixed.
3. Compressive strength
The compressive strength values (in MPa) are summarized in Table 2 and Fig. 6. ECPR had the highest compressive strength (76.67 ± 25.67 MPa), followed by WRPR, RTMX, and EZMX (38.39 ± 7.25, 38.17 ± 2.50, and 4.07 ± 0.60 MPa, respectively). Significant differences (p < 0.0083) were observed in terms of compressive strength among RTMX, EZMX, and ECPR and among WRPR, EZMX, and ECPR. In contrast, RTMX and ECZR showed no significant difference regarding their compressive strength (p > 0.0083).

Schematic representation of the compressive strength results (n = 5).
p-value obtained from the Kruskal-Wallis test and the Mann-Whitney U test with Bonferroni correction.
n = number of measurements. The data are shown as mean ± SD values of 5 samples.
The lowercase letters indicate statistically significant differences (p < 0.0083).
RTMX: RetroMTA®; EZMX: Endocem® MTA Zr; WRPR: Well-RootTM PT; ECPR: Endocem® MTA premixed.
Discussion
The properties of CSCs can only be examined after they are set, and the setting time of these cements is influenced by various factors, such as temperature, humidity, experimental environment, mixing method, quantity of water used, and packing force [25,26]. The ideal setting time for pulp capping materials can vary depending on the specific material and clinical application. However, a setting time of around 10 - 15 minutes is often considered desirable for pulp capping procedures [27]. The setting time should be balanced with other critical properties, such as compressive strength and biocompatibility [28].
The setting time of EZMX and RTMX showed no significant difference (p > 0.0083) in our study, which is consistent with a previous study where both materials had a setting time of 150 seconds [29]. EZMX is characterized by its rapid setting time due to the small pozzolanic cement particles present, which enable it to set quickly without the need for a chemical accelerator [30-32]. Notably, RTMX forms calcium zirconia complexes that change the setting chemistry and reduce the setting time [33,34]. Moreover, the presence of calcium carbonate in RTMX contributes to significantly reducing the initial and final setting times and improving the mechanical strength [35].
In contrast, the premixed-type CSCs (WRPR and ECPR) had longer setting times. Based on previous studies, detailed information on the components of these premixed-type products is not presented by the manufacturer, but it can be assumed that hydroxypropyl cellulose or propylene glycol is added to improve maneuverability [13]. These additives can lead to a reduction in the amount of available water for the hydration reaction, which further prolongs the initial setting time [36-38].
While some studies suggest that longer setting times might result in increased material strength and reduced solubility, prolonging the setting time for pulp capping materials may have negative consequences, such as increased chair time, patient discomfort, and higher risks of material displacement or contamination before the material has fully set [5,39-43]. Notably, the chair time is an important factor for children undergoing dental treatment owing to their specific characteristics, such as shorter attention spans and a tendency to become anxious or restless. Thus, an optimal setting time for pulp capping materials should balance these competing factors to provide effective treatment while minimizing potential risks. The findings of this study could contribute to the choice of the appropriate material for a specific clinical situation.
High solubility results in permanent seal failure due to loss of material [44,45]. Therefore, the proper solubility with adequate dissolution resistance in dentinal fluid, body fluids, or oral fluids is required to maintain the sealing ability [21,25]. According to ISO 6876, the solubility of CSC within 3% was considered acceptable. All materials, except for WRPR, satisfy this condition [46]. In WRPR, the use of polypropylene glycol as a solvent may have contributed to its high solubility value [47]. In a previous study, it was suggested that incompletely dried glycol is retained during the solubility test, which results in a higher solubility value. This finding is consistent with the results of our study [21]. However, in another study, Ashi et al. [48] reported a solubility value of 1% for WRPR, which is inconsistent with our findings. This discrepancy may be attributed to variations in the experimental methods used for measuring the samples [49]. Unlike Ashi et al. [48] who measured solubility as the difference between the initial and final weights of the samples, we followed ISO 6876 guidelines and measured the dissolved amount by weighing the beaker before and after the dissolution process. WRPR could potentially increase the risk of bacterial leakage and endodontic failure due to the high solubility associated with additives in the matrix [50]. In clinical use, high solubility needs to be considered. However, owing to the lack of studies measuring the solubility of WRPR at present, additional studies on the solubility of WRPR should be conducted.
In contrast, ECPR and EZMX demonstrated low solubility. The low solubility may be attributed to their composition and setting reaction. The ECPR is reported to have washout resistance due to the presence of anti-washout compositions, such as hydroxypropyl methylcellulose and dimethyl sulfoxide [51-53]. In a previous study, the solubility value of ECPR was measured at 0.28%, which is consistent with the results of our study [21]. For EZMX, the rapid setting of pozzolan cement enhances its washout resistance [51,53]. Thus, the solubility of CSCs can be affected by various factors, such as the setting time and composition. Further studies are needed to evaluate the effects of different compositions on solubility, in order to gain a better understanding of CSCs.
The compressive strength test is used to simulate the stress that may result from forces clinically applied to a restorative, baseliner, or core build-up material owing to the fact that most masticatory forces are compressive in nature [54]. Thus, to be used as a base material, the cement must possess sufficient compressive strength to withstand masticatory forces [55-57].
Although no requirements have been presented for calcium silicate-based materials, ISO 9917-1 [24] indicated that the standard compressive strength of dental hydraulic cements should be ≥ 50 Mpa. Only ECPR met this condition in the present study. The ECPR was found to contain calcium sulfate, which can enhance compressive strength by accelerating the hydration reaction [32].
Conversely, the compressive strength of EZMX in the present study was 4.07 MPa, which was the lowest among all tested materials (p < 0.0083). Previous studies also observed a low compressive strength of 8.9 Mpa for EZMX [15]. These indicated that EZMX can be selected for sites where high compressive strength is not required, such as anterior teeth, and that this material cannot be used as a base [58].
In the present study, the compressive strengths of WRPR and RTMX were 38.39 ± 7.25 MPa and 38.17 ± 2.50 MPa, respectively, which were lower than the values reported in previous studies [15,56]. This difference could be attributed to the existence of voids inside the specimen. The various factors, including the sample size and shape, time for hydration, technique of mixing, preparation method, powder-liquid ratio, pressure used when compacting the samples in the mold, and temperature, influence compressive strength measurement [54,56,59]. According to a previous study, WRPR contains polyethylene glycol and polypropylene glycol [38]. These components were added to improve maneuverability, but other studies reported that their inclusion led to an increase in flowability and setting time and a decrease in compressive strength [13,36]. These findings of this study are expected to provide useful information for the selection of CSC materials and for guiding further study.
However, this study has several limitations. First, the number of specimens was insufficient, and the lack of established standards for calcium silicate-based materials led to the use of experimental methods based on ISO 6876 and ISO 9917-1 guidelines, which may result in varying clinical relevance. Second, no direct comparisons of cytotoxicity and cell differentiation were performed. Third, it could not precisely replicate the oral environment, leading to potential variations when the materials are used in actual clinical settings.
Hence, further in vivo experiments are needed to simulate clinical situations, including cytotoxicity and cell differentiation, and an official International Organization for Standardization (ISO) for testing CSCs is needed.
Conclusion
This study compared the setting time, solubility, and compressive strength of four types of CSCs. The premixed type CSCs (WRPR and ECPR) exhibited longer setting times compared to the powder-liquid mix type CSCs (EZMX and RTMX). The highest solubility was observed in WRPR, followed by RTMX and ECMX. ECPR showed the lowest solubility, with significant differences with WRPR. The highest compressive strength was observed in ECPR, followed by WRPR and RTMX, with no significant difference between the latter two. In contrast, EZMX showed the lowest compressive strength. High solubility in WRPR and low compressive strength in EZMX can lead to treatment failure, so this should be taken into consideration when selecting materials based on the specific clinical situation.
Although ECPR has a longer setting time than EZMX and RTMX, it is a promising material in that it has improved solubility and compressive strength, and the working time can be shortened due to the characteristics of premixed CSC.
Notes
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Funding information
This work was supported by the Basic Science Research Program funded by the Ministry of Education (NRF-2022R1I1A1A01069606).