|
 |
| J Korean Acad Pediatr Dent > Volume 50(4); 2023 > Article |
|
Abstract
ACKNOWLEDGMENTS
Fig.┬Ā1.
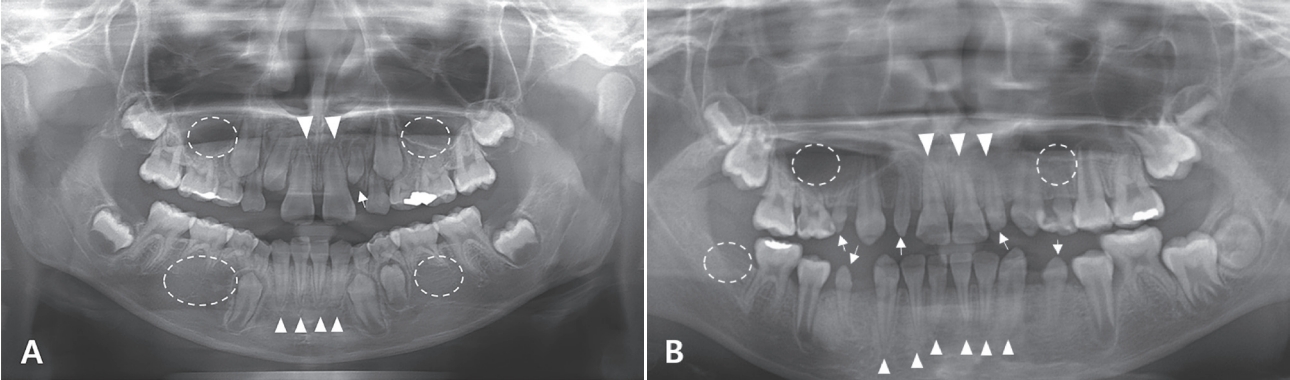
Fig.┬Ā2.

Table┬Ā1.
| Characteristics | All | AD < 3 years | 3 years Ōēż AD < 6 years | 6 years Ōēż AD | p value | |
|---|---|---|---|---|---|---|
| Gender (male : female) | 69 : 56 | 34 : 31 | 18 : 12 | 17 : 13 | ||
| Age at diagnosis (years) | 2.92 (3.39) | 1.58 (0.83) | 4.17 (0.94) | 8.96 (2.42) | < 0.0001 | |
| Age at treatment completion (years) | 5.42 (3.42) | 3.50 (2.30) | 6.04 (1.90) | 10.04 (2.47) | < 0.0001 | |
| Age at evaluation (years) | 10.67 (4.58) | 8.75 (3.79) | 10.67 (4.60) | 13.75 (4.34) | < 0.0001 | |
| Age at radiation (years) | 3.38 (2.91) | 2.50 (1.89) | 4.92 (1.10) | 9.33 (2.20) | < 0.0001 | |
| Treatment duration (years) | 1.33 (1.89) | 1.75 (2.16) | 1.42 (1.78) | 0.96 (0.87) | 0.0040* | |
| Total number of chemotherapeutic classes | 4.00 (1.54) | 4.00 (1.58) | 5.00 (1.52) | 4.00 (1.45) | 0.2211 | |
| Total number of chemotherapeutic agents | 7.00 (2.91) | 8.00 (3.15) | 8.00 (2.60) | 6.00 (2.48) | 0.0678 | |
| Radiation dose (Gy) | 40.80 (19.02) | 36.00 (15.78) | 54.00 (23.61) | 57.60 (16.94) | 0.2576 | |
| Diagnosis | ||||||
| Acute lymphoblastic leukemia | 36 (28.80) | 16 (24.62) | 12 (40.00) | 8 (26.67) | 0.2928 | |
| Acute myelogenous leukemia | 9 (7.20) | 3 (4.62) | 2 (6.67) | 4 (13.33) | 0.3086 | |
| Lymphoma | 18 (14.40) | 6 (9.23) | 3 (10.00) | 9 (30.00) | 0.0202ŌĆĀ | |
| Brain tumor | 18 (14.40) | 6 (9.23) | 8 (26.67) | 4 (13.33) | 0.0781 | |
| Sarcoma | 12 (9.60) | 6 (9.23) | 3 (10.00) | 3 (10.00) | 0.9894 | |
| Wilms tumor | 4 (3.20) | 4 (6.15) | 0 (0.00) | 0 (0.00) | 0.1485 | |
| Retinoblastoma | 5 (4.00) | 5 (7.69) | 0 (0.00) | 0 (0.00) | 0.0904 | |
| Others | 23 (18.40) | 19 (29.23) | 2 (6.67) | 2 (6.67) | 0.0050ŌĆĀ | |
| Treatment modalities | ||||||
| Chemotherapy alone | 52 (41.6) | 29 (44.62) | 11 (36.67) | 12 (40.00) | 0.2120 | |
| Chemotherapy + RT | 19 (15.2) | 7 (10.77) | 7 (23.33) | 5 (16.67) | ||
| Chemotherapy + HSCT | 35 (28.0) | 16 (24.62) | 7 (23.33) | 12 (40.00) | ||
| Chemotherapy + RT + HSCT | 19 (15.2) | 13 (20.00) | 5 (16.67) | 1 (3.33) | ||
Table┬Ā2.
| Dental abnormalities | All | AD < 3 years | 3 years Ōēż AD < 6 years | 6 years Ōēż AD | p value |
|---|---|---|---|---|---|
| Normal | 42 (33.60) | 13 (20.00) | 9 (30.00) | 20 (66.67) | 0.0001* |
| Abnormal root development only | 17 (13.60) | 3 (4.62) | 10 (33.33) | 4 (13.33) | 0.0007* |
| Tooth agenesis only | 6 (4.80) | 3 (4.62) | 1 (3.33) | 2 (6.67) | 0.8291 |
| Microdontia only | 19 (15.20) | 15 (23.08) | 2 (6.67) | 2 (6.67) | 0.0384* |
| Tooth agenesis + Abnormal root development | 6 (4.80) | 4 (6.15) | 0 (0.00) | 2 (6.67) | 0.3675 |
| Microdontia + Abnormal root development | 5 (4.00) | 2 (3.08) | 3 (10.00) | 0 (0.00) | 0.1220 |
| Tooth agenesis + microdontia | 8 (6.40) | 7 (10.77) | 1 (3.33) | 0 (0.00) | 0.1006 |
| Tooth agenesis + Microdontia + Abnormal root development | 22 (17.60) | 18 (27.69) | 4 (13.33) | 0 (0.00) | 0.0034* |
Table┬Ā3.
| Characteristics | All | AD < 3 years | 3 years Ōēż AD < 6 years | 6 years Ōēż AD | p value | |
|---|---|---|---|---|---|---|
| Tooth agenesis | No | 83 (66.40) | 33 (50.77) | 24 (80.00) | 26 (86.67) | 0.0005* |
| Yes | 42 (33.60) | 32 (49.23) | 6 (20.00) | 4 (13.33) | ||
| Microdontia | No | 71 (56.80) | 23 (35.38) | 20 (66.67) | 28 (93.33) | < 0.0001* |
| Yes | 54 (43.20) | 42 (64.62) | 10 (33.33) | 2 (6.7) | ||
| Abnormal root development | No | 75 (60.00) | 38 (56.25) | 13 (43.33) | 24 (80.00) | 0.0140* |
| Yes | 50 (40.00) | 27 (43.75) | 17 (56.67) | 6 (20.00) | ||
| Oligodontia | No | 29 (69.05) | 18 (58.06) | 7 (100.00) | 4 (100.00) | 0.0282* |
| Yes | 13 (30.95) | 13 (41.94) | 0 (0.00) | 0 (0.00) |
Table┬Ā4.
| Characteristics | All (n = 125) | AD < 3 years (n = 65) | 3 years Ōēż AD < 6 years (n = 30) | 6 years Ōēż AD (n = 30) | p value |
|---|---|---|---|---|---|
| Tooth agenesis | 1.67 (3.28) | 2.92 (4.12) | 0.40 (0.97) | 0.23 (0.68) | < 0.0001* |
| Microdontia | 1.94 (2.76) | 2.71 (2.80) | 2.07 (3.20) | 0.17 (0.65) | < 0.0001* |
| Abnormal root development | 3.99 (7.15) | 3.34 (5.77) | 8.83 (10.31) | 0.57 (1.28) | < 0.0001* |
| V-shaped root | 3.61 (6.94) | 3.18 (5.81) | 7.93 (9.98) | 0.20 (0.61) | < 0.0001* |
| U-shaped root | 0.41 (1.40) | 0.22 (0.87) | 0.90 (2.32) | 0.20 (0.61) | 0.0796 |
Table┬Ā5.
| Characteristics | Tooth agenesis | Microdontia | Abnormal root development | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p value | No | Yes | p value | No | Yes | p value | ||
| Gender | ||||||||||
| Male | 49 (59.04) | 20 (47.62) | 0.2253 | 37 (52.11) | 32 (59.26) | 0.4261 | 44 (58.67) | 25 (50.00) | 0.3398 | |
| Female | 34 (40.96) | 22 (52.38) | 34 (47.89) | 22 (40.74) | 31 (41.33) | 25 (50.00) | ||||
| Age of Diagnosis | ||||||||||
| AD < 3 years | 33 (39.76) | 32 (76.19) | 0.0005* | 23 (32.39) | 42 (77.78) | < 0.000* | 38 (50.67) | 27 (54.00) | 0.0140* | |
| 3 years Ōēż AD < 6 years | 24 (28.92) | 6 (14.29) | 20 (28.17) | 10 (18.52) | 13 (17.33) | 17 (34.00) | ||||
| 6 years Ōēż AD | 26 (31.33) | 4 (9.52) | 28 (39.44) | 2 (3.70) | 24 (32.00) | 6 (12.00) | ||||
| Diagnosis | ||||||||||
| No brain tumor | 72 (86.75) | 35 (83.33) | 0.6076 | 60 (84.51) | 47 (87.04) | 0.6898 | 71 (94.67) | 36 (72.00) | 0.0004* | |
| Brain tumor | 11 (13.25) | 7 (16.67) | 11 (15.49) | 7 (12.96) | 4 (5.33) | 14 (28.00) | ||||
| Use of heavy metal agents | ||||||||||
| No | 60 (72.29) | 16 (38.1) | 0.0002* | 49 (69.01) | 27 (50.00) | 0.0310* | 55 (73.33) | 21 (42.00) | 0.0004* | |
| Yes | 23 (27.71) | 26 (61.9) | 22 (30.99) | 27 (50.00) | 20 (26.67) | 29 (58.00) | ||||
| Multiple class of chemotherapeutic agents | ||||||||||
| < 4 class | 50 (60.24) | 13 (30.95) | 0.0020* | 40 (56.34) | 23 (42.59) | 0.1279 | 45 (60.00) | 18 (36.00) | 0.0086* | |
| Ōēź 4 class | 33 (39.76) | 29 (69.05) | 31 (43.66) | 31 (57.41) | 30 (40.00) | 32 (64.00) | ||||
| Head and neck radiation | ||||||||||
| No | 8 (40.00) | 7 (38.89) | 0.9442 | 5 (29.41) | 10 (47.62) | 0.2536 | 4 (40.00) | 11 (39.29) | 0.9684 | |
| Yes | 12 (60.00) | 11 (61.11) | 12 (70.59) | 11 (52.38) | 6 (60.00) | 17 (60.71) | ||||
| Radiation dose | ||||||||||
| < 40 Gy | 9 (45.00) | 8 (44.44) | 0.9726 | 7 (41.18) | 10 (47.62) | 0.2536 | 4 (40.00) | 13 (46.43) | 1.0000 | |
| Ōēź 40 Gy | 11 (55.00) | 10 (55.56) | 10 (58.82) | 11 (52.38) | 6 (60.00) | 15 (53.57) | ||||
| HSCT | ||||||||||
| No | 29 (34.94) | 25 (59.52) | 0.0088* | 28 (39.44) | 26 (48.15) | 0.3301 | 22 (29.33) | 32 (64.00) | 0.0001* | |
| Yes | 54 (65.06) | 17 (40.48) | 43 (60.56) | 28 (51.85) | 53 (70.67) | 18 (36.00) | ||||
| Surgery | ||||||||||
| No | 27 (32.53) | 22 (52.38) | 0.0318* | 24 (33.80) | 25 (46.30) | 0.1564 | 24 (32.00) | 25 (50.00) | 0.0434* | |
| Yes | 56 (67.47) | 20 (47.62) | 47 (66.20) | 29 (53.70) | 51 (68.00) | 25 (50.00) | ||||
| Treatment modality | ||||||||||
| Chemo | 40 (48.19) | 12 (28.57) | 0.0039* | 30 (42.25) | 22 (40.74) | 0.0048* | 45 (60.00) | 7 (14.00) | < 0.000* | |
| Chemo + RT | 14 (16.87) | 5 (11.9) | 13 (18.31) | 6 (11.11) | 8 (10.67) | 11 (22.00) | ||||
| Chemo + HSCT | 23 (27.71) | 12 (28.57) | 24 (33.8) | 11 (20.37) | 20 (26.67) | 15 (30.00) | ||||
| Chemo + RT + HSCT | 6 (7.23) | 13 (30.95) | 4 (5.63) | 15 (27.78) | 2 (2.67) | 17 (34.00) | ||||
| Treatment duration | ||||||||||
| < 1.5 years | 51 (61.45) | 27 (64.29) | 0.7568 | 52 (73.24) | 26 (48.15) | 0.0041* | 49 (65.33) | 29 (58.00) | 0.4070 | |
| Ōēź 1.5 years | 32 (38.55) | 15 (35.71) | 19 (26.76) | 28 (51.85) | 26 (34.67) | 21 (42.00) | ||||
Table┬Ā6.
| Characteristics | Overall dental abnormalities | Tooth agenesis | Microdontia | Abnormal root development | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | ||
| Gender | |||||||||||||
| male | reference | ||||||||||||
| female | 1.15 | 0.54 - 2.45 | 0.7229 | 1.59 | 0.75 - 3.35 | 0.2267 | 0.75 | 0.37 - 1.53 | 0.4265 | 1.42 | 0.69 - 2.92 | 0.3405 | |
| Diagnosis age (years) | |||||||||||||
| < 3 | 6 | 2.32 - 15.52 | 0.0002* | 6.30 | 1.98 - 20.09 | 0.0019* | 25.56 | 5.56 - 117.08 | < .0001* | 2.84 | 1.02 - 7.89 | 0.0451* | |
| 3 Ōēż and < 6 | 3.5 | 1.20 - 10.20 | 0.0217* | 1.63 | 0.41 - 6.47 | 0.4911 | 7.00 | 1.38 - 35.47 | 0.0188* | 5.23 | 1.66 - 16.51 | 0.0048* | |
| Ōēź 6 | reference | ||||||||||||
| Diagnosis | |||||||||||||
| No brain tumor | reference | ||||||||||||
| Brain tumor | 1.78 | 0.55 - 5.78 | 0.3413 | 1.31 | 0.47 - 3.67 | 0.6083 | 0.81 | 0.29 - 2.26 | 0.6902 | 6.90 | 2.12 - 22.49 | 0.0013* | |
| Use of heavy metal agents | |||||||||||||
| No | reference | ||||||||||||
| Yes | 3.06 | 1.30 - 7.20 | 0.0104* | 4.24 | 1.93 - 9.31 | 0.0003* | 2.23 | 1.07 - 4.64 | 0.0323* | 3.80 | 1.78 - 8.12 | 0.0006* | |
| Multiple class of chemotherapeutic agents | |||||||||||||
| < 4 class | reference | ||||||||||||
| Ōēź 4 class | 1.31 | 0.62 - 2.79 | 0.4809 | 3.38 | 1.54 - 7.43 | 0.0025* | 1.74 | 0.85 - 3.55 | 0.1292 | 2.67 | 1.27 - 5.59 | 0.0094* | |
| Head and neck radiation | |||||||||||||
| No | reference | ||||||||||||
| Yes | 0.55 | 0.09 - 3.31 | 0.5172 | 1.05 | 0.29 - 3.86 | 0.9442 | 0.46 | 0.12 - 1.77 | 0.2573 | 1.03 | 0.24 - 4.50 | 0.9683 | |
| Radiation dose | |||||||||||||
| < 40 Gy | reference | ||||||||||||
| Ōēź 40 Gy | 0.91 | 0.17 - 4.77 | 0.9118 | 1.02 | 0.28 - 3.68 | 0.9726 | 0.77 | 0.2 - 2.80 | 0.6915 | 0.77 | 0.18 - 3.34 | 0.7260 | |
| HSCT | |||||||||||||
| No | reference | ||||||||||||
| Yes | 3.22 | 1.40 - 7.40 | 0.0059* | 2.74 | 1.28 - 5.88 | 0.0097* | 1.43 | 0.70 - .92 | 0.3308 | 4.28 | 2.00 - 9.18 | 0.0002* | |
| Surgery | |||||||||||||
| No | reference | ||||||||||||
| Yes | 1.30 | 0.60 - 2.84 | 0.5098 | 2.28 | 1.07 - 4.88 | 0.0334* | 1.69 | 0.82 - 3.49 | 0.1578 | 2.13 | 1.02 - 4.44 | 0.0449* | |
| Treatment modalities | |||||||||||||
| Chemotherapy | reference | ||||||||||||
| Chemotherapy + RT | 2.59 | 0.82 - 8.25 | 0.1066 | 1.19 | 0.36 - 3.98 | 0.7772 | 0.63 | 0.21 - 1.92 | 0.4148 | 8.84 | 2.64 - 29.64 | 0.0004* | |
| Chemotherapy + HSCT | 3.13 | 1.20 - 8.15 | 0.0198* | 1.74 | 0.67 - 4.50 | 0.2538 | 0.63 | 0.25 - 1.54 | 0.3066 | 4.82 | 1.70 - 13.65 | 0.0030* | |
| Chemotherapy + RT+ HSCT | 7.87 | 1.65 - 37.56 | 0.0097* | 7.22 | 2.26 - 23.10 | 0.0009* | 5.11 | 1.49 - 17.54 | 0.0095* | 54.64 | 10.31 - 289.57 | < 0.0001* | |
| Treatment duration (years) | |||||||||||||
| < 1.5 | reference | ||||||||||||
| Ōēź 1.5 | 1.94 | 0.86 - 4.38 | 0.1126 | 0.89 | 0.41 - 1.91 | 0.7569 | 2.95 | 1.39 - 6.23 | 0.0047* | 1.37 | 0.65 - 2.85 | 0.4076 | |
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 97 View
- 41 Download
- ORCID iDs
-
Jihyun Lee

https://orcid.org/0000-0001-6093-4102Hyung-Jun Choi

https://orcid.org/0000-0002-3315-6912Jaeho Lee

https://orcid.org/0000-0002-1556-3485Je Seon Song

https://orcid.org/0000-0001-8620-5629Chung-Min Kang

https://orcid.org/0000-0001-7813-3741 - Related articles
-
Assessment of Risk Factors for Developmental Defects of the Enamel in Preterm2023 May;50(2)
Assessment of Dental Noise Environment of a Pediatric Dentist2021 May;48(2)
Assessment of Predicting Factors for Pediatric Sleep Disordered Breathing2020 November;47(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print