|
 |
| J Korean Acad Pediatr Dent > Volume 44(2); 2017 > Article |
|
міИл°Э
мЭі мЧ∞кµђмЭШ л™©м†БмЭА м•РмЭШ нХШмХЕ м†ИмєШмЧРмДЬ мГЭлђЉнХЩм†Б кіСнЩФмЧР лМАнХЬ л∞ЬмЬ° лЛ®к≥Дл•Љ нГАмЮДнЕМмЭілЄФл°Ь мИШл¶љнХШлКФлН∞ мЮИлЛ§.
лІємґЬ кЄЄмЭік∞А мЄ°м†ХлРШмЧИк≥† м°∞мІБнХЩм†Б м†ИнОЄк≥Љ лѓЄмДЄлЛ®мЄµміђмШБкЄ∞(microscopic computerized tomography) лЛ®л©імЬЉл°Ь м†ИмєШ л∞ЬмЬ°мЭШ 4лЛ®к≥Д (1) preodontoblast, (2) dentin matrix secretion, (3) enamel matrix secretion, кЈЄл¶ђк≥† (4) enamel calcificationмЭД нЩХмЭЄнХШмШАлЛ§.
м•РмЭШ нХШмХЕ м†ИмєШмЧРмДЬ міЭ лІємґЬ мЖНлПДлКФ 600 ¬± 70 ќЉm/day (mean ¬± SD; n = 12) мШАлЛ§. л≤ХлЮСмІИ лґДлєД кЄЄмЭілКФ м°∞мІБнХЩм†Б м†ИнОЄмЧРмДЬ 4.59 ¬± 0.75 mm, л∞©мВђмД†нХЩм†Б лЛ®л©імЧРмДЬ 3.64 ¬± 0.63 mmмШАк≥† мЭіл•Љ к∞Бк∞Б мЖНлПДл°Ь нЩШмВ∞нХШл©і 180.4 ¬± 30.0 hours, 145 ¬± 25 hours мЬЉл°Ь лВШнГАлВђлЛ§(n = 24).
мЭілЯђнХЬ к≤∞к≥ЉлУ§мЭА м•РмЭШ м†ИмєШмЭШ мГЭлђЉнХЩм†Б кіСнЩФк∞А мЭЉмЦілВШлКФ л∞ЬмЬ° 4 лЛ®к≥Дк∞А лЛ® л©∞мє† лІМмЧР мЭіл£®мЦімІДлЛ§лКФ к≤ГмЭД м†ЬмЛЬнХЬлЛ§. мЭіл≤И лПЩлђЉ мЛ§нЧШмЧ∞кµђмЭШ к≤∞к≥ЉлКФ л∞ЬмЬ° м§СмЭЄ мєШл∞∞мЭШ лє†л•Є кіСлђЉнЩФ к≥Љм†ХмЭД мєШмХД л∞ЬмЬ° кЄ∞к∞ДмЭШ лґДмДЭмЭД нЖµнХі мЭінХінХ† мИШ мЮИлЛ§лКФ к≤ГмЧР мЭШмЭШк∞А мЮИлЛ§.
Abstract
The aim of this study was to estimate time of biomineralization in developmental stages of rat lower incisors.
Eruption length was measured. Four stages of incisor development were identified on histologic and microscopic computerized tomography (micro-CT) sections: (1) preodontoblast, (2) dentin matrix secretion, (3) enamel matrix secretion, and (4) enamel calcification.
The overall eruption rate of the rat lower incisor was 600 ¬± 70 ќЉm/day (mean ¬± SD; n = 12). The length of the enamel secretion was 4.59 ¬± 0.75 mm in histologic section, was 3.64 ¬± 0.63 mm in radiographic section, which converts to 180.4 ¬± 30.0 hours, 145 ¬± 25 hours respectively (n = 24).
These findings suggested that the four biomineralizing developmental stages of the rat incisor took only several days. The significance of this animal study was to provide understanding for the rapid biomineralization process of developing rat tooth germ by analysis of tooth forming period.
The incisors of rodents have only a thin layer of enamel (about 0.1 mm) on the labial surface. Their incisors are morphologically distinct from human incisors, but their enamel and dentin are histologically the same with those of human, which can be very helpful for researching and understanding the development process in human teeth [1]. Moreover, rat incisors grow throughout the animalвАЩs lifetime. When secretary proteins induce formation of hard tissues, rat incisors rapidly calcify and erupt to perform masticatory function.
This model is helpful for studying teeth generation, since tooth-related cells can be observed sequentially in all stages of development within a tooth in order. Also, the secretion and calcification processes that form the dentin and enamel can be observed [2]. The innermost part of the rat incisor has a cervical loop, a starting point of tooth development, and the other end has an incisal edge. This histologic observation reflects an orderly process in which various undifferentiated cells of teeth development gradually become differentiated after a period of proliferation, and the differentiated odontoblasts and ameloblasts then secrete dentin and enamel matrix. The successive calcification of the secreted substrates can be clearly observed. Suga [3] classified the hard tissue formation process broadly into three periods: (1) dentin matrix secretion, (2) enamel matrix secretion, and (3) enamel calcification.
Since the constantly erupting incisor of rodents is a single mass, the tooth eruption rate must be the same as the formation and movement rate of the dental tissues that develop in the alveolus. Thus, the length of histochemical biologic change in the dental tissue can be calculated into the time period. The tooth eruption rate must be identical to the total proliferation rate of tooth-forming cells as well as the formation rate of dental hard tissues [4-6]. Unlike rat incisors, the eruption of human teeth can be divided into two major stages, pre- and post-emergent eruption [7]. The eruption of human teeth seems to be related to their root formation [8]. So, preemergent eruption, teeth germs do not show any movement toward the oral cavity until root development begins. A previous study reported the eruption rate of human teeth ranged from 0.08 to 0.32 mm/wk, during post-emergent eruption [9].
Ameloblasts located on the labial side of the rat lower incisor are useful for research on the proliferation potential and movement rate of these cells, because the processes of differentiation, maturation, and degeneration can be clearly observed in this region [2,10-12]. Hwang and Tonna [11] measured the eruption rate and movement rate of ameloblasts of the mouse incisor, and reported that they were identical. Other researchers have subsequently agreed with this finding [13-16]. The hypothesis underlying this study was that the natural, continually growing rat incisor maintained a constant balanced length. In other words, the eruption rate of incisor in rodents is the same as the proliferation rate of tooth forming cells, rate of dental matrix formation, rate of calcification in enamel and dentin, and rate of attrition.
While previous studies compared the eruption rate of rodentsвАЩ teeth and the movement rate of related cells [17-19], there are few studies that investigated the period and time required for completion of the biomineralization process of the tooth structure. Moreover, it is hard to find metric data regarding the length at each differentiation stage of tooth-related cells in the rat incisor with conversion into time based on eruption rates.
The subjects of this study were 12 male Sprague-Dawley rats (NTacSam:SD, Orient Bio, Korea) weighing between 270 and 320 g that were born and bred in sterilized environment. An adaptation period of 1 week was allowed in which the rats were maintained under the following living conditions: 22 ± 1°C, 55% humidity, 12-/12-h light/dark cycle, and a standard diet and sufficient water. Male rats were used in order to exclude the effect of sex hormone on the tooth eruption rate. The rats were randomly divided into three groups and a notch on the rat incisor was formed in each group on a separate day to minimize time difference.
This experiment was conducted with the approval of the Institutional Animal Care and Use Committee of the medical college at Yonsei University. All authors have no financial and personal relationships that could influence their work.
Rats were anesthetized via an intraperitoneal injection of 0.1 mg/100 g of a mixture of Zoletil (tiletamine hydrochloride, zolazepam hydrochloride, 50 mg/ml; Virbac, France) and Rompun (xylazine hydrochloride, 23.32 mg/ml; Bayer, Korea) at a ratio of 3:2. After placing the rat on an experiment board, its mouth was opened with a master pincette and a notch was created at the lowest height of the labial gingival margin of the mandible with a saw-toothed laboratory blade. This blade was made by creating five or six small semicircular notches after the incisal edges of the blade was removed with a high-speed, needle-shaped diamond bur (Fig. 1A).
The rats were sacrificed after 7 days, and total 24 left and right mandibles from 12 rats were separated and stored in 4% paraformaldehyde (PFA) for 1 day. The mandibles were placed so that the curved incisor plane was parallel to the floor, and the outer sagittal plane of the mandible was photographed through a stereomicroscope with a built-in digital camera (Olympus SZX12, Olympus, Japan). Olysia BioReport software (Olympus Australia, Australia) was used to measure the lengths of the curves on the photographs along the labial side of the incisor from the notch to the gingival margin (Fig. 1B). An Excel spreadsheet (Microsoft, USA) was used to calculate the mean ± SD value of the length of erupted tooth over the 7-day period. This length was converted into the eruption rate based on the elapsed time. The mandibles that were photographed and then measured were stored again in PFA in preparation for histologic and radiologic observations.
The various stages of lower-incisor development were assessed in histologic sections obtained from the 12 left mandibles. The mandibles were first decalcified with 10% ethylenediaminetetraacetic acid (EDTA) for 4 weeks, gradually dehydrated in a series of ethyl alcohol solutions in the standard way, and then embedded with paraffin in a position which the outer side of the mandible faced the floor. The outer side of the mandible was placed toward the floor so that it could be cut parallel with the section of incisor. The angle of the embedded specimens was adjusted to obtain the maximum area of incisor section with the microtome (RM2235, Leica Biosystems, Germany). Sections were cut at a thickness of 7 ќЉm, and the slides were stained with hematoxylin-eosin (H-E) and MassonвАЩs trichrome stain. Cellular changes in H-E-stained sections were observed using an optical microscope, and the trichrome-stained sections were used to observe secreted dentin and enamel matrix substrates. The length of each phase was measured using NIH Image-J software (http://rsb.info.nih.gov/ij,USA). The teeth were classified by observing the sections histologically, and the length of each stage was measured under a microscope.
The dental mesenchymal cells adjacent to epithelial cells develop into polarized pre-odontoblasts first, then continuously develop into secretory odontoblasts, which secret dentin matrix and are responsible for the dentin formation. The dental epithelial precursor cells located in the cervical loop migrate to the basal layer around the outside of the loop. The basal epithelial cells polarize and elongate to become pre-ameloblasts, and then differentiate into secretory ameloblasts, which secrete enamel matrix proteins and attract minerals to initiate enamel biomineralization [20]. The developmental stages of the rat lower incisor could be divided into four stages in this study according to PingpingвАЩs method [21]: (1) presecretory odontoblast: before secretion of dentin matrix, (2) presecretory ameloblast: Secretion of dentin matrix, (3) secretory ameloblast: Secretion of enamel matrix, and (4) enamel calcification: reaching final length of enamel thickness (Fig. 2A) [21].
The calcification stages of the hard tissue of the incisors were studied in dehydrated right mandibles using microscopic computerized tomography (micro-CT), measuring from the posterior wall of cervical loop to the following four radiographically observed stages according to YanвАЩs method [20]: (1) initiation of dentin calcification, (2) initiation of enamel matrix secretion, (3) endpoint of enamel secretion, and (4) starting point of almost complete calcified enamel. The hard tissues of the desired teeth were reconstructed in three dimensions from the radiologic findings (Fig. 2B).
Right mandibles stored in 4% PFA were imaged with micro-CT (Skyscan 1072, Belgium), and the basic size and axis length of the incisor were measured using NIH Image-J software. Furthermore, the length of each phase of enamel secretion, deposition, and calcification was identified with DataViewer (Skyscan; Fig. 3).
The eruption rate of the rat incisor was calculated as 600 ¬± 70 ќЉm/day, 25 ¬± 3 ќЉm/hour. Observation of the specimens of lower incisors revealed that the osseous tissues directly enclosed the rearmost point of the cervical loop, which was considered the starting point of tooth development (Fig. 2e, Fig. 4B). This rearmost point was also clearly distinguishable on radiography. The secretion of dentin substrate occurred at a later time (Fig. 2f, Fig. 4C), followed by secretion of the enamel substrate (Fig. 2h, Fig. 4D, Fig. 4E). The starting point of enamel substrate secretion tended to coincide with the predentin starting point. With subsequent calcification of the enamel, the color of enamel substrate, which had been initially stained an eosinophilic color, became lighter and faded, probably due to decalcification of the calcified portion by EDTA (Fig. 4F). H-E staining was difficult to distinguish between the enamel and dentin initial secretion point because they showed similar colors. Therefore, Masson's trichrome staining was carried out in order to distinguish them. Collagens before dentin to be calcified were stained blue and amelogenins of enamel were stained red by Masson's trichrome staining (Fig. 4G).
Based on these histologic findings, the starting point of the secretory ameloblast zone coincided with the point at which the layer began to thicker when ameloblasts facing enamel formed TomвАЩs process. The ameloblast maturation stage began when TomeвАЩs process disappeared and the cells shortened, and ended where the thickness of the enamel increased and eventually became consistent. The morphometric data (mean ¬± SD values) was calculated (Table 1).
The basic size of the lower incisor was also analyzed (Table 2).
The lengths of the zone of dentin formation, the start and endpoints of enamel substrate secretion, and the endpoint of enamel calcification were measured (Table 3).
The eruption length of the rat incisor was measured, and times taken for differentiation of undifferentiated cells, deposition of dentin and enamel, and calcification of the enamel were calculated. Lower-incisor development was observed using both radiologic and histologic methods; differences were found between the findings obtained with the two methods.
Smith and Warshawsky [12] reported that it is almost impossible to locate a section axis that includes the entire root apex to the incisal edge in rat incisors. The mandibular symphysis on both sides of the mandible is agglutinated by fibrous tissue comprising fibrocartilage and intercrossing ligaments. This fibrous tissue allows the mandible to rotate up to 40¬Ї about its axis, thus separating the two lower incisors, enabling the rats to masticate [22]. Hence, mesiodistal and mediolateral curves appear on same surface, so it is difficult to obtain a section including the entire area from a root apex to incisal edge. This may lead to measurement errors when an oblique section is used, because different starting points were chosen. In this study, the specimen angle was finely adjusted in the microtome so as to locate the optimum tooth axis, and the specimens included the root apex and at least the mesial side of the first molar tooth. The mesial side of the first molar tooth is where the enamel maturation stage ends, and includes all of the tissues under observation. However, because all specimens have slightly different angles in cross section, errors could have been made measuring the location of the cells and hard tissues. On the other hand, the micro-CT images allowed the visualization of various cross sections in three dimensions, although a precise observation at the cellular level was not possible; therefore, these images were used for analyzing the differentiation stage.
There are several studies on the eruption rate of the rat lower incisor (Table 4). The factors thought to affect incisor eruption rate include age, root excision, neurectomy, diet, stress, hormones, drugs, occlusion, wear, fluoride, sleep, and pulp cutting. The first predictable factor is the damage of the marginal gingiva caused by creating a notch. It could have induced gingival recession, resulting in overestimation of the eruption length. The second factor is measurement made at curvature. It is measurement of our study that was differed from most previous studies in which the straight distance was measured. The third factor is diet; if harder food was provided to the animals, it may have induced a faster eruption rate [29]. In previous studies, the feed was stored in the presence of moisture at room temperature. However, the feed could be drier and harder, due to careful storage in recent studies. Therefore, the feed used in this study could have been harder than those used by other researchers.
Several studies have been published on the location and arrival time of incisors at each differentiation stage. Chase [4] argued that the last stage of incisor maturation was completed at about one-third of the distance from the end of the incisal edge to the gingival margin. In other research on the mouse, ameloblast proliferation and maturation were completed after 24 hours, and the ameloblasts recessed 9 days thereafter maturation [11]. Smith and Warshawsky [12] found that ameloblasts and odontoblasts moved at rates of 651 and 631 ќЉm/day, respectively, in the rat lower incisors. In the present study, the durations of lower-incisor differentiation at the preodontoblast secretion, preameloblast secretion, ameloblast secretion, and enamel calcification phases, observed by micro-CT, were 74.6, 153.9, 145.4, and 148.8 hours, respectively; the corresponding durations on histologic observation were 16.8, 20.8, 183.7, and 101.9 hours. Although there are clear differences in the findings obtained between the two methods, we believe that because precise observation at a cellular level is possible in the histologic sections, the histologic observations must be closer to the actual values, although the radiologic method can provide additional information not available with histologic results.
In this study, the eruption rate of rat incisors was measured, development stages were determined by histologic or radiographic observations of regions from the cervical loop to the incisal edge, the length of each stage was measured and converted into time based on the eruption rate of the teeth, and the time required for each stage of the toothвАЩs main development was determined. The results of this study could provide important basic data to improve our understanding of time required for biologic tooth development and calcification.
This study developed the timetable of biomineralization corresponding developmental stages of lower incisors in rats. Enamel and dentin matrix of rats are histochemically the same as those of humans. Accordingly, based on the present study, if the thickness of human enamel and dentin is known, it is possible to estimate the time required for biomineralization in human. Although further study should be needed, this data can be used as important clinical information.
The tooth development is an important topic in pediatric dentistry and pediatric dentists see developing tooth germs on radiographs every day. Because information of tooth biomineralization is extremely limited, it is difficult for a dentist to decide the stage of dental formation and biomineralization. It seems that human teeth are in the alveolar bone for 6-8 years from birth and mineralize for many years before eruption. In this study, the biomineralization in rats was not completed in a long period of time but in a matter of days. Since the structure of human and rat teeth are almost the same, the clinical significance can be found in the light of odontogenesis in pediatric dentistry. Thus, this result can improve understanding of tooth development in human.
This study determined the biomineralization period of each stage of lower-incisor development in rats, in which the teeth developmental process is similar to humans. The eruption rate (i.e., the increase in length from the labial gingival margin to the notch over time) was about 600 ќЉm/day (about 25 ќЉm/hour). The length of the zone of enamel secretion was about 4.6 mm, and it took about 184 hours (about 7.7 days) to complete (as assessed histologically). The length of the zone of enamel calcification was about 3.7 mm, and it took about 148 hours (about 6.2 days) to complete (as assessed radiographically). This suggested that the four developmental biomineralization stages of the rat incisor took only several days.
The findings from this animal study could help to understand the rapid biomineralization process of developing tooth germ in rats by analyzing tooth forming period.
Fig. 1.
Blade modification and length measurement. (A) Modified blade with several grooves created using a high-speed diamond bur. This simple and effective method was used to carve a sharp notch on the labial side of the enamel surface of the rat lower incisor. The notch was made on the lowest free gingival margin. (B) Measurement of the amount of eruption amount of the rat lower incisor, marked as вАШXвАЩ (upper arrow, notch; lower arrow, labial gingival margin; scale bar = 2 mm).
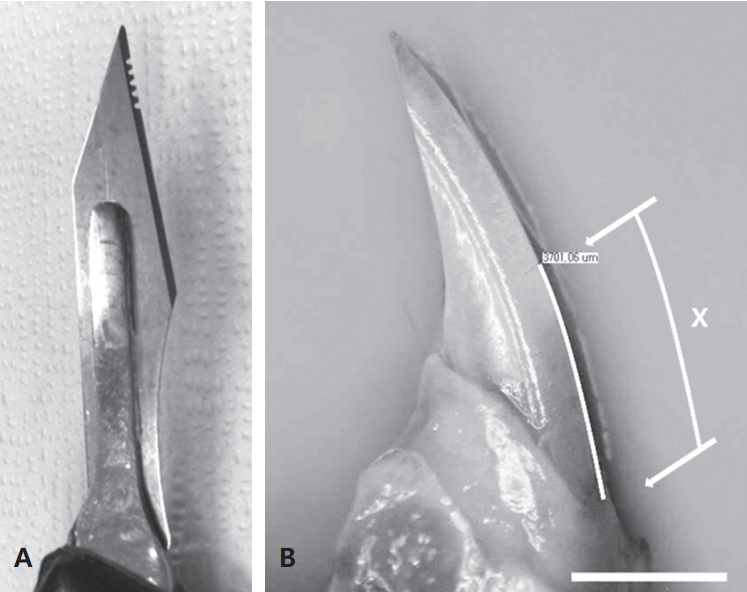
Fig. 2.
Measurements of length according to the development stage in the rat lower incisor. (A) Schematic drawing of a histologic section viewed longitudinally (dentin, green; enamel, yellow; pulp, red; ameloblast, pink). The histological classification according to PingpingвАЩs method. a. zone of presecretory odontoblasts, b. zone of presecretory ameloblasts, c. zone of secretory ameloblasts, d. zone of enamel calcification (B) Longitudinal section of an incisor using micro-CT (scale bar = 2 mm). The classification of calcification stages according to YanвАЩs method. e. posterior wall of the cervical loop, f. initiation of dentin calcification, g. initiation of enamel matrix secretion, h. endpoint of enamel secretion, i. point of almost completely calcified enamel.

Fig. 3.
Micro-CT view of the rat lower incisor. (A) Coronal section (a. labiolingual thickness of the incisor. b. mesiodistal thickness of the incisor, c. enamel thickness). (B) Longitudinal section (curved line, labial surface on longitudinal section indicating the entire arch length of the tooth). C: Cross section of A was taken at C. Enamel in region C is calcified and right before eruption.

Fig. 4.
Longitudinal section of a rat mandibular incisor. (A) Longitudinal section showing cellular changes of odontoblasts and ameloblasts, and matrix secretion of dentin and enamel. Dentin and enamel in pink and purple, respectively. The black lines indicate the same point as the displayed portion in Fig. 2. (B) Cervical loop and initiation of the presecretory odontoblast zone. (C) Initiation of the presecretory ameloblast zone. (D) Long secretory ameloblasts starting to produce enamel. (E) Shorter, mature ameloblasts and calcification of the enamel. (F) The zone of mature enamel appears translucent due to decalcification. (G) Dentin and enamel in blue and red, respectively (A: H-E stain, scale bar = 20 µm; B-F: H-E stain, scale bar = 10 µm; G: trichrome stain, scale bar = 10 µm).
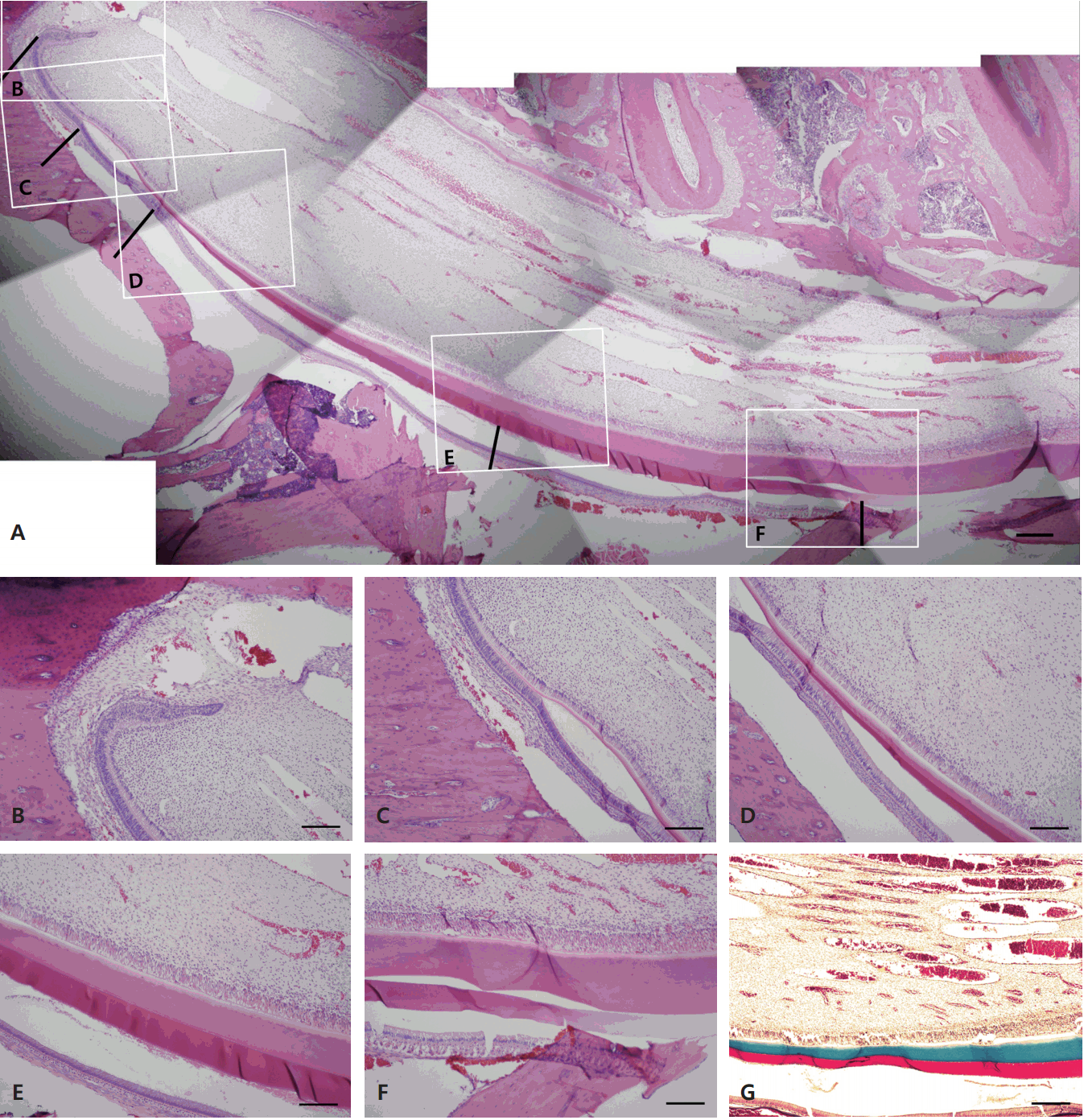
Table 1.
Lengths of the zones of odontogenesis and amelogenesis in the rat lower incisor on histologic sections, and the time required to complete each zone (n = 24)
Table 2.
Measurements on cross-sectional micro-CT (n = 6)
| Dimension | Measured value (µm) |
|---|---|
| Mean ± SD | |
| Labiolingual thickness | 2160 ± 55 |
| Mesiodistal thickness | 1350 ± 24 |
| Enamel thickness | 133 ± 10 |
Table 3.
Lengths of the zones of odontogenesis and amelogenesis in the rat lower incisor on radiography, and the time required to complete each zone (n = 24)
References
1. Pinzon RD, Kozlov M, Burch WP : Histology of rat molar pulp at different ages. J Dent Res, 46:202-208, 1967.


2. Shore RC, Kolokuris I, Robinson C, Kirkham J : Immunohistochemical investigation of epidermal growth factor receptor expression during periods of accelerated rat incisor eruption. Arch Oral Biol, 37:389-393, 1992.


3. Suga S : Amelogenesis. Some histological and histochemical observations. Int Dent, 9:394-420, 1959.
4. Chase SW : The Interrelation of Maturation and the Histogenesis of Hypoplasia in Dental Enamel. Proc Dent Centenary Cel, 425-431, 1940.
5. Michaeli Y, Weinreb MM, Zajicek G : Role of Attrition and Occlusal Contact in the Physiology of Rat Incisors: V. Life Cycle of Inner Enamel Epithelial Cells at Various Rates of Eruption. J Dent Res, 51:960-963, 1972.


7. Steedle JR, Proffit WR : The pattern and control of eruptive tooth movements. Am J Orthod, 87:56-66, 1985.


8. Carlson H : Studies on the rate and amount of eruption of certain human teeth. Am J Orthod Oral Surg, 42:78-91, 1944.


9. Smith RG : A clinical study into the rate of eruption of some human permanent teeth. Arch Oral Biol, 25:675-681, 1980.


10. Chiba M : Cellular proliferation in the tooth germ of the rat incisor. Arch Oral Biol, 10:707-718, 1965.


11. Hwang WSS, Tonna EA : Autoradiographic analysis of labeling indices and migration rates of cellular component of mouse incisors using tritiated thymidine (H3TDR). J Dent Res, 44:42-53, 1965.


12. Smith CE, Warshawsky H : Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. Anat Rec, 183:523-561, 1975.


14. Reith EJ, Cotty VF : Autoradiographic studies on calcification of enamel. Arch Oral Biol, 7:365-372, 1962.


15. Robinson C, Hiller CR, Weatherell JA : Uptake of P-labelled phosphate into developing rat incisor enamel. Calcif Tissue Int, 15:143-152, 1974.

16. Zajicek G, Michaeli Y, Weinreb MM : Kinetics of the inner enamel epithelium in the adult rat incisor during accelerated eruption. Cell Prolif, 5:35-39, 1972.

17. Law KT, Lee CK, King NM, Rabie AB : The relationship between eruption and length of mandibular incisors in young rats. Med Sci Monit, 9:BR47-53, 2003.

18. Burn-Murdoch RA : The length and eruption rates of incisor teeth in rats after one or more of them had been unimpeded. Eur J Orthod, 21:49-56, 1999.


19. Skobe Z, Heeley JD, Stern DN, et al. : Comparison of rates of enamel synthesis in impeded and unimpeded rat incisors. J Dent Res, 72:46-50, 1993.


20. Zhang Y, Kim SO, DenBesten PK, et al. : Multiple effects of the cellular prion protein on tooth development. Int J Dev Biol, 55:953-960, 2011.


21. He P, Zhang Y, DenBesten PK, et al. : Ameloblast differentiation in the human developing tooth: effects of extracellular matrices. Matrix Biol, 29:411-419, 2010.



22. Addison WHF, Appleton Jr. JL : The structure and growth of the incisor teeth of the albino rat. J Morph, 26:43-96, 1915.

23. Chiba M, Takizawa K, Ohshima S : Dose-response effects of colchicine and vinblastine on unimpeded eruption rates of the rat mandibular incisor. Arch Oral Biol, 25:115-119, 1980.


24. Lavelle CL : The effect of age on the eruption rate of the incisor teeth of the rat (Rattus norvegicus). J Anat, 104:109-116, 1969.


26. Sturman GD : A study of the eruption rate of the rat mandibular incisor. Yale J Biol Med, 30:137-148, 1957.


27. Weinmann JP, Wessinger GD, Reed G : Correlation of Chemical and Histological Investigations on Developing Enamel. J Dent Res, 21:171-182, 1942.

- TOOLS
-
METRICS

-
- 4 Crossref
- 0 Scopus
- 4,649 View
- 132 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print