타액 및 혈액오염 시 유치 지르코니아 기성관 내면의 세척 방법에 따른 결합강도의 비교
Comparison of Bonding Strength by Cleaning Method of Pediatric Zirconia Crown Contaminated with Saliva or Blood
Article information
Abstract
본 연구의 목적은 타액이나 혈액에 오염된 유치 지르코니아 기성관의 내면을 다양한 세척 방법으로 세척 한 뒤, 레진 시멘트와의 결 합력을 비교하고, 열 순환이 결합력에 미치는 효과를 평가하였다.
지르코니아 기성관의 내면과 유사한 상태의 지르코니아 절편(5 mm x 3 mm)을 아크릴릭 레진에 매몰하였다(n=180). 이 중 160개의 시편을 무작위로 두 군으로 나누어 타액 오염과 혈액 오염을 시행하였다. 양성 대조군은 오염 과정을 거치지 않고 resin cement로 접착을 시행하였고, 음성 대조군은 오염 후 dental unit chair 상의 물로 20초간 세척 후 10초간 건조를 시행하였다. 타액 오염된 시편과 (n=60) 혈액 오염된 시편(n=60)은 각각 세 가지 방법의 세척을 시행하였다. 37% 인산 젤, 상용 세척제(Ivoclean), 2.5% NaOCl을 각 시편에 20초간 적용 후 10초간 물로 세척하고 건조를 시행하였다. 이 후 모든 시편(n=160)에 접착을 시행하였다. 대조군과 각 실험군을 모두 절반으로 나누어 절반은 바로 전단응력을 측정하였고, 절반은 열 순환 과정 후 전단응력을 측정하였다.
타액오염과 혈액오염을 시행한 군 모두에서, 양성 대조군과 상용 세척제, 2.5% NaOCl으로 세척한 군에서 통계학적으로 유의한 차이는 존재하지 않았다. 음성 대조군과 37% 인산 젤로 세척한 군의 전단 결합 강도는 양성 대조군과 비교하였을 때, 유의하게 감소하였다. 열 순환 시행 시 타액 오염을 시행한 군과 혈액 오염을 시행한 모든 군에서 열 순환을 시행하지 않은 군보다 통계적으로 유의하게 낮은 전단 응력 간도를 나타내었다.
유치 지르코니아 기성관 내부의 타액 오염이나 혈액 오염 시, 상용 세척제나 2.5% NaOCl로 세척을 시행하면 원래의 전단응력 값을 얻을 수 있다.
열 순환 시행 시 모든 군에서 유의하게 낮은 전단 응력 값을 보였다.
Trans Abstract
The objective of this study was to compare the shear bonding strength of zirconia after cleaning the crown contaminated by saliva or blood and determine the effect of thermocycling. 180 specimens were embedded in acrylic resin. 20 Specimens in the positive control group were bonded with resin cement without contamination. 20 Specimens in the negative control group were washed with water for 20 seconds and then dried for 10 seconds. 120 Specimens contaminated by saliva or blood were cleaned by using three cleaning methods: 37% phosphoric acid gel, commercial cleanse, and 2.5% NaOCl. All samples were bonded with resin cement and divided into two subgroups: One was not aged, and the other was tested with 30,000 thermocycling. In both groups contamination by saliva and blood, no statistically significant difference was not found in control, groups cleansed by commercial cleanser and 2.5% NaOCl. When the groups cleansed with water and 37% phosphate gel were compared with the control, significantly low shear bond strength was shown. Thermocycling group showed statistically significantly low shear bond stress compared to the groups without thermocycling. When zirconia was contaminated by saliva or blood, its original shear bond strength could be obtained if it was cleaned with commercial cleanser or 2.5% NaOCl.
Ⅰ. Introduction
Zirconia, also known as ceramic oxide, has been used in dental treatment for various purposes such as endodontic treatment as root canal post, fixed denture, and implant abutment due to its excellent strength, aesthetics, and good biocompatibility[1-3]. However, unlike silica based all - ceramic, zirconia cannot be attached using bonding agents since zirconia does not contain silica[4]. For this reason, air abrasion in the inside of zirconia and silanization treatment are used for adhesion between zirconia restoration and bonding agents[5].
The long term adhesive strength is influenced by moisture content, ceramic characteristics such as surface roughness, bonding agent characteristics such as composition or viscosity, and various factors involved in the adhesion process[6-9]. A practical obstacle in zirconia adhesion is contamination by saliva or blood, resulting in weakening adhesion between inner surface of zirconia and cement[10]. To remove such contamination, a variety of methods have been suggested, such as the use of acetone, 70% isopropanol, 96% ethanol, air abrasion, 37% phosphate gel, 2% chlorhexidine, 5% NaOCl, or water spray[11,12]. Yang et al .[10] have reported that cleansing the inside of zirconia contaminated by saliva with isopropanol and water cannot completely remove the organic coating formed by saliva[13-16].
Recently, Ivoclean (Ivoclar Vivadent, Schaan, Liechtenstein), a cleanser for the inside of zirconia, has been introduced in the dental market. The manufacturer claims that the cleanser can effectively remove contamination by saliva that causes organic coating on various restorations including zirconia ceramic through a simple spray, cleansing, and air - drying method. However, no study has reported the cleansing effect of such solution on primary teeth contaminated by saliva or blood.
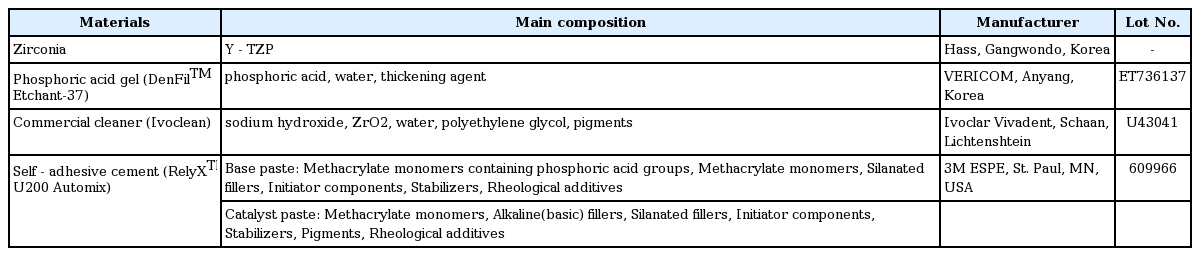
Components of pediatric zirconia crown and the zirconia crown for permanent teeth are the same as those of Yttriastabilized tetragonal zirconia polycrystalline (Y-TZP). However, their manufacturing processes are different. Pediatric zirconia crown is produced through injecting zirconium dioxide and yttrium oxide into a mold under high temperature. The crown’s inside pattern is determined by the mold’s shape used in injection. On the contrary, zirconia crown used for permanent teeth is manufactured by milling Y-TZP block using CAD/CAM. This causes huge differences in inner pattern and surface characteristic such as surface roughness between crowns for primary teeth and those for permanent teeth (Fig. 1).
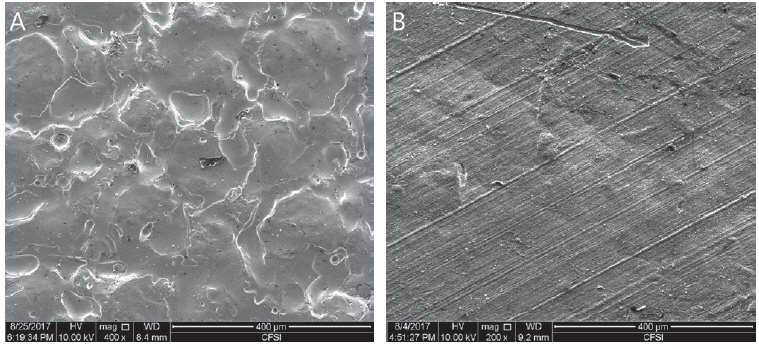
Scanning Electron Microscopy images (×400). (A) Inner side of NuSmile® ZR crown, (B) Inner side of Zirconia crown for permanent tooth.
Many previous studies have elucidated that thermocycling process during adhesion of zirconia with resin cement can significantly reduce shear bond strength[11,17,18]. In in vitro study, techniques such as undergoing thermocycling process in different temperature or storing water under constant temperature are generally used to evaluate shear bond strength. Changing temperature plays a significant role in altering adhesion strength of dental restorations[19,20]. However, few studies have investigated the effect of cleansing methods on thermocycling when pediatric zirconia crown is contaminated by blood or saliva. Therefore, the objective of this study were: (1) to compare bonding strength with resin cement of zirconia crown after cleaning the crown contaminated by saliva or blood, and (2) to determine the effect of thermocycling treatment on shear bond strength. The first null hypothesis is cleaning method does not affect shear bond strength. The second null hypothesis is that thermocycling process does not affect shear bond strength.
Ⅱ. Materials and Methods
This study was performed after obtaining approval from Institutional Review Board (IRB) of Gangneung-Wonju National University Dental Hospital (IRB No.: 2016 - 022).
1. Materials
In this study, self-adhesive cement RelyXTM U200 Automix was used as adhesive material while 37% phosphate gel (Den-FilTM Etchant-37), commercial cleanser (Ivoclean), and 2.5% NaOCl were used as cleansers (Table 1).
2. Manufacturing Zirconia Disk Specimen
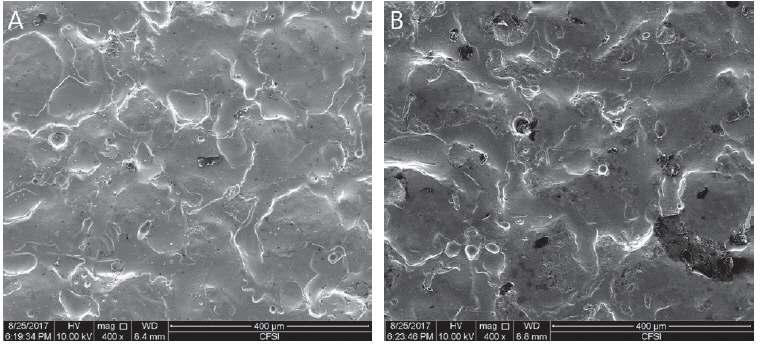
To obtain zirconia specimens with surface same as the inside of pediatric zirconia crown (NuSmile® ZR crown), we requested HASS (Gangneung, South Korea), a manufacturer of pediatric zirconia crown under business agreement with NuSmile Pediatric Crowns (Houston, TX, USA), to produce specimens. These zirconia specimens were molded under pressure into disk shape with diameter of 8 mm and height of 2 mm. They were then prepared by pressureless sintering at temperatures of 1500℃ for 2 hours. Upper punch of the mold used in compression molding was prepared to have the same surface as the inner surface of zirconia crown (Fig. 2). Zirconia specimens were placed in the middle of 25 × 20 × 5 mm mold and casted by self-cured resin (Jet Teeth Shade™ Powder: Lang Dental Mfg Inc, Wheeling, IL, USA). A total of 180 zirconia specimens were randomly divided into 20 specimens for each group with different cleaning methods or the control group.
3. Contamination Process of Specimens
Stimulated saliva and venous blood were collected from healthy non - smoking subjects after fasting for two hours. A total of 160 specimens were randomly divided into saliva or blood groups. 80 of these specimens were immersed in saliva while the other 80 specimens were immersed in blood for 60 seconds. They were then washed with water from dental unit chair and air dried.
4. Cleaning Procedure and Adhesion
A total of nine experimental groups were used in this study: 1) Group Ⅰ (Positive Control, PC): No contamination; 2) Group Ⅱ (Saliva contamination - Ivoclean cleaning, S-IV): After saliva contamination, Ivoclean was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; 3) Group Ⅲ (Saliva contamination - 2.5% NaOCl cleaning, S-Na): After saliva contamination, 2% NaOCl was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; 4) Group Ⅳ (Saliva contamination - 37% phosphoric acid gel cleaning, S-PA): After saliva contamination, 37% phosphate gel was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; 5) Group Ⅴ (Saliva contamination - Negative control, S-NC): After saliva contamination, specimens were washed with water from dental unit chair for 20 seconds followed by air drying for 10 seconds; 6) Group Ⅵ (Blood contamination - Ivoclean cleaning, B-IV): After blood contamination, Ivoclean was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; 7) Group Ⅶ (Blood contamination - 2.5% NaOCl cleaning, B-Na): After blood contamination, 2% NaOCl was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; 8) Group Ⅷ (Blood contamination - 37% phosphoric acid gel cleaning, B-PA): After blood contamination, 37% phosphate gel was applied to the surface of specimens for 20 seconds. They were then washed with water for 10 seconds followed by air drying for 10 seconds; and 9) Group Ⅸ (Blood contamination - Negative control, B-NC): After blood contamination, specimens were washed with water from dental unit chair for 20 seconds followed by air drying for 10 seconds. After the cleaning process, self-adhesive resin cement was bonded to each specimen. When applying cement, teflon mold with internal diameter of 5 mm and height of 3 mm was used to allocate the same amount of cement.
5. Thermocycling and Storage
Each experimental group was divided into two subgroups (subgroup A, subgroup B). Subgroup A was stored in wetting thermostat at 37℃ for 24 hours without thermocycling before measuring shear bond strength. Subgroup B was stored in wetting thermostat at 37℃ for 24 hours followed by thermocycling (5℃ and 55℃ for 30 seconds, 30,000 times)[21].
6. Shear Bond Strength Test
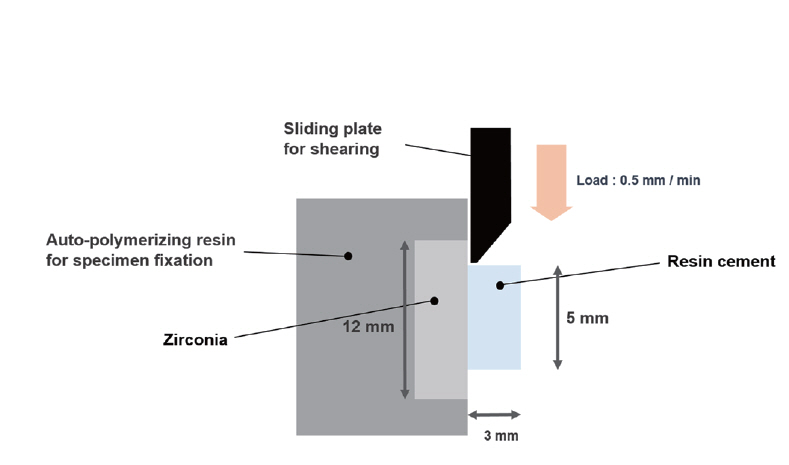
Shear bond strength of specimen was measured using Universal Testing Machine (R&B Inc., Daejeon, South Korea). Measuring speed was set at 0.5 mm/min. Maximum stress (MPa) was measured when adhesion between specimen and cement was fractured (Fig. 3).
7. Fracture Pattern Classification
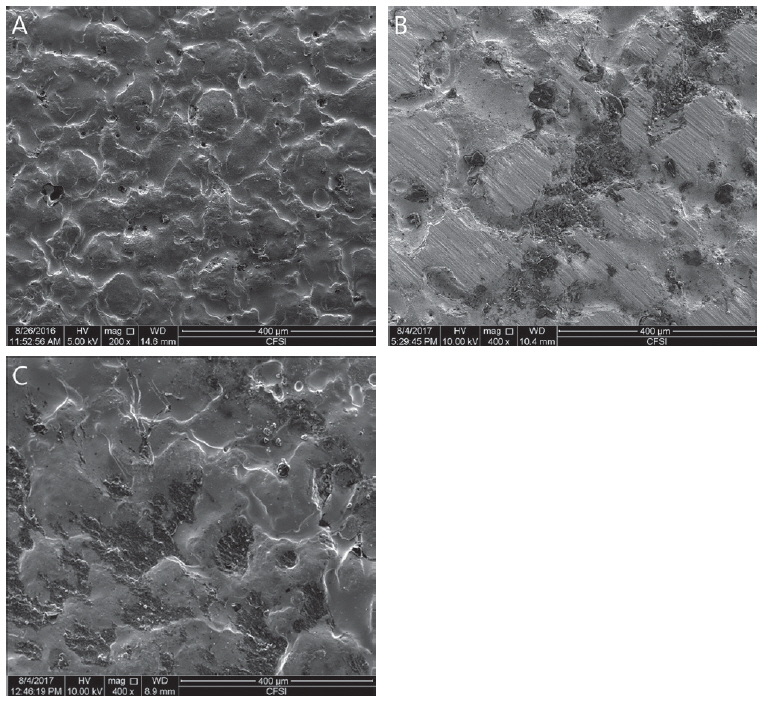
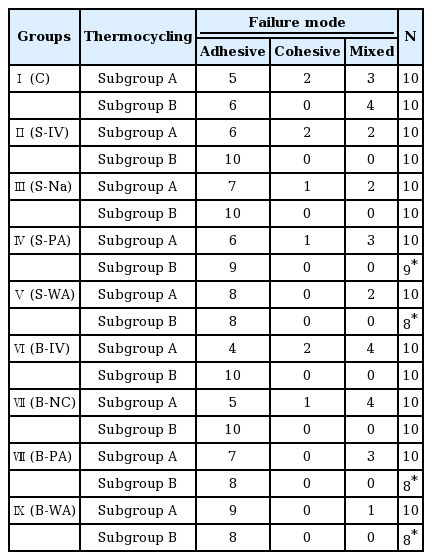
After measuring shear bond strength, the fractured surface was coated by 10 nm platinum particle. The coated surface was observed with a Scanning Electron Microscope (SEM) at magnification of 200 and 400 and classified by fracture patterns. The surface was classified as adhesive failure (failure of adhesion on interface), cohesive failure (failure due to lack of cohesive strength), and mixed failure (failure showing mixed pattern of adhesive and cohesive failures) (Fig. 4).
8. Statistical Analysis
Mean and standard deviation were calculated for saliva contaminated group and blood contaminated group. To verify normality of data, Shapiro - Wilk test was conducted. Normality of the data was confirmed. To determine whether shear bond strength was different depending on cleaning methods, one-way analysis of variance (ANOVA) and post-hoc test were conducted. Independent sample t-test was used to compare differences before/after conducting thermocycling. Statistical analyses were performed using SPSS 23.0 (IBM Corp., Chicago, IL, USA) with alpha level of 0.05. Tukey’s HSD was used for post-hoc test.
Ⅲ. Results
1. Shear Bond Strength of Saliva or Blood Contaminated Specimen depending on Cleaning Methods
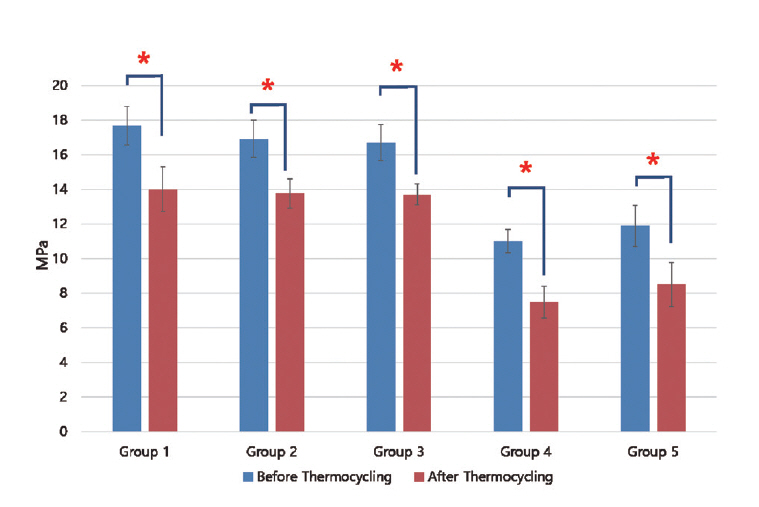
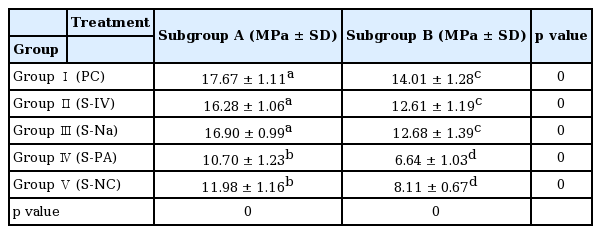
Results of shear bond strength of saliva or blood contaminated specimens depending on cleaning methods are summarized in Tables 2 and 3. When non - thermocycling treated experimental groups were compared, no contamination group showed the highest shear bond strength. Among saliva or blood contaminated groups, water cleaning group showed the lowest shear bond strength. Shear bond strength of specimen in the non - contaminated group was not significantly different from that of Ivoclean or 2.5% NaOCl cleaned group. Shear bond strength of specimen in phosphate cleaned group was significantly lower compared to that in Ivoclean or 2.5% NaOCl cleaned group, although it was significantly different from that of the non - cleaning group. All experimental groups showed significant reductions in shear bond strength after thermocycling treatment (Fig. 5, 6).
Mean shear bond strength (MPa) with standard deviation according to cleaning method after saliva contamination
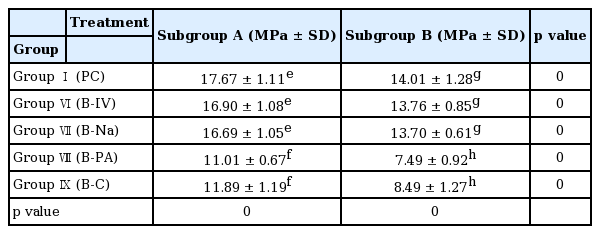
Mean shear bond strength (MPa) with standard deviation according to cleaning method after blood contamination
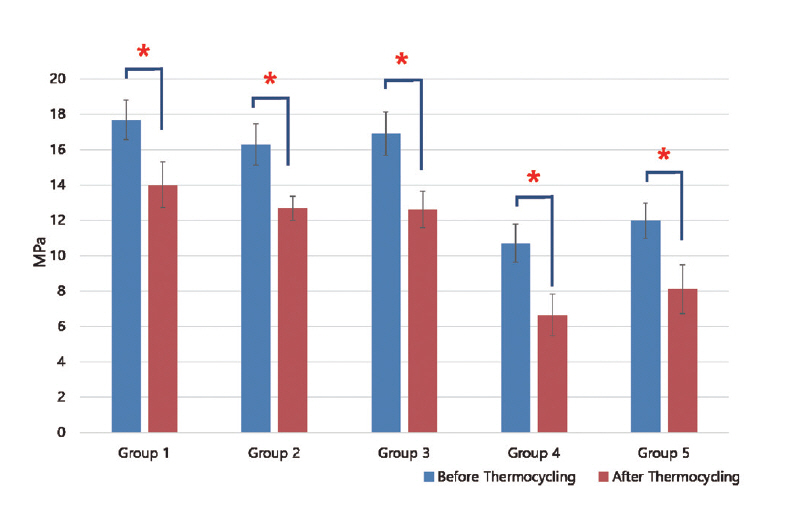
Mean and standard deviation of shear bond strength of zirconia discs contaminated with saliva after various cleaning methods and control group. Significant at p < 0.05 level (*).
2. Fracture Pattern Classification
Results of fracture pattern classification are shown in Table 4. Before thermocycling treatment, adhesive failure was dominant in all experimental group. For non - contaminated group and saliva or blood contaminated group cleaned with Ivoclean or 2.5% NaOCl, 10 to 20% of cohesive failure was observed. After thermocycling treatment, cohesive fracture was observed in all experimental groups. In 10 - 20% of saliva or blood contaminated group cleaned with phosphate or water, resin cement naturally detached. These specimens were excluded for shear bond strength measurement or SEM observation.
Ⅳ. Discussion
In this study, zirconia specimens imitating pediatric zirconia crown were used. After they were contaminated with saliva or blood, their shear bond strengths were measured depending on cleaning methods. Significant reduction in shear bond strength was observed for saliva or blood contaminated group cleaned with water or 37% phosphate gel. Thus, the first null hypothesis that cleaning method does not affect shear bond strength is rejected. Gale and Darvell[22] have insisted that 10,000 times thermocycling process is clinically equivalent to one year of use. In the present study, 30,000 times of thermocycling was implemented based on their research. After the thermocycling process, statistically significant reduction in shear bond strength was observed in all experimental groups. Therefore, the second null hypothesis that thermocycling process does not affect shear bond strength is also rejected.
Luthy et al .[23] have reported that clinically acceptable minimum adhesion strength between zirconia and adhesive materials is 10 - 13 MPa. All experimental groups without undergoing the thermocycling process showed average shear bond strength of more than 10 MPa. After the thermocyling process, experimental groups cleansed by water or phosphate showed shear bond strength of less than 10 MPa.
Materials and recommended methods for applying resin cement adhesion to ceramic are usually based on laboratory measurement such as tensile bond strength, micro tensile bond strength, and shear bond strength. Shear bond strength as the most commonly used method has been proved to be reliable[6,7]. Therefore, it was chosen in this study.
Saliva contains organic materials such as saliva protein, enzyme, bacteria, food debris, and inorganic materials such as mineral ions[10]. If saliva protein is adhesive to restoration materials, non - bacterially acquired enamel pellicle with thickness of 10 - 20 nm can be formed within few minutes. It is almost impossible to avoid saliva contamination of zirconia restoration agents since protein layer consisting of protein, enzyme, glucoprotein, and other macro - molecules will form immediately under increased protein transferring[10,24]. When contaminated by blood, macro - molecules such as components containing high amounts of protein, fibrinogen, and thrombocyte may form a layer at dentin surface, thus inhibiting infiltration of adhesive materials into microtubules and affecting adhesive strength[25]. Air abrasion is the most commonly used method to clean contamination caused by saliva or blood. Although air abrasion can effectively remove contamination and restore bonding strength similar to that without contamination, surface damage due to particles related to air abrasion may weaken the internal strength of zirconia. Zhang et al .[13] have insisted that, although damage caused by air abrasion is not immediately observed, it has negative effect on fatigue strength of zirconia restoration. Phark et al .[7] have reported that air abrasion can weaken shear bond strength in the long term by removing micropores in zirconia. Many other studies have also claimed that damage by particles can affect the long term strength of zirconia[10,15-18,21,26]. For those reasons, air abrasion was not included in this study. Further studies are needed to determine changes in bond strength of zirconia in long term.
Of the three cleaning methods, 37% phosphate cleaning resulted in the lowest shear bond strength of zirconia after saliva or blood contamination. After thermocycling process, the phosphate cleaning method resulted in the lowest shear bond strength, although it had clinically acceptable shear bond strength before thermocycling, consistent with a previous study showing that saliva - contaminated zirconia cleaned with phosphate showed remarkably reduced shear bond strength[27]. This indicates that cleaning using 37% phosphate gel may alter surface energy and reduce bonding characteristics, thus decreasing shear bond strength, although it can remove organic contaminants in zirconia[10].
For saliva and blood contamination, both Ivoclean and 2.5% NaOCl showed good cleaning results and high shear bond strength. Shear bond strength values were also clinically acceptable even after thermocycling. It is currently unclear how these two materials clean the inside of contaminated zirconia. It is known that 2.5% NaOCl can remove organic materials and proteins in smear layer of dentin as a non - specific proteolytic agent. Akin et al . have reported that cleaning method with 0.5% NaOCl can successfully restore shear bond strength. Clinically used concentration of NaOCl is 0.5 - 2.5%[28]. Therefore, 2.5% NaOCl was used in this study. Saliva is composed of phosphate salts groups in forms of phospholipids that bond to the inside of zirconia actively. According to the manufacturer, Ivoclean consists of zirconia, water, polyethylene glycol, sodium hydroxide, and other additives. This indicates that phosphate salt contaminants are more likely to be absorbed by Ivoclean than the inside of zirconia, thereby cleaning the inner surface of zirconia.
One limitation of this study was that quantitative analysis was not conducted to determine the level of contamination inside the zirconia or how much each cleaning method removed contaminants. This study focused on changes in shear bond strength after cleaning the inner surface of zirconia contaminated by saliva or blood. Further studies are needed to elucidate changes in physical properties of the internal surface of zirconia.
Ⅴ. Conclusion
This study performed cleaning of saliva or blood contaminated zirconia specimens imitating the inside of pediatric zirconia crown. For saliva or blood contaminated specimens, adhesive strength between zirconia specimen and resin cement was not significantly different between non - contamination group and Ivoclean or 2.5% NaOCl cleaned group. After thermocycling process, shear bond strengths of specimens cleansed with Ivoclean or 2.5% NaOCl were reduced. However, they still showed clinically acceptable levels of strength.
Saliva or blood contaminated specimens cleansed with 37% phosphate gel showed statistically significant reduction in shear bond strength. Its shear bond strength was not significantly different from that of negative control. Before thermocycling process, these specimens had clinically acceptable values of bond strength. However, after thermocycling, these specimens failed to have clinically acceptable values of bond strength. After thermocycling, significant reduction in shear bond strength was observed in samples with all cleaning methods. Shear bond strengths were not significantly different among samples with different cleaning methods after the thermocylcing process.