유치 줄기세포에 대한 다양한 규산칼슘계 재료의 세포독성
Cytotoxicity of Various Calcium Silicate-based Materials with Stem Cells from Deciduous Teeth
Article information
Abstract
이 연구의 목적은 유치 줄기세포에 대해 다양한 Calcium silicate-based material (CSM)의 세포독성을 비교하고 평가하는 것이다. Retro MTA® (RM), EZ-SealTM (EZ), ENDOCEM Zr® (EN)의 powder를 세포 배지를 이용하여 용출시키고 filtering 하였다. 유치 줄기세포가 다양한 농도의 용출액 하 배양되었다. MTS assay를 통해 CSM 용출액이 세포 증식에 미치는 영향을 분석하였고, Flow cytometry analysis를 통해 세포 표현형의 변화 여부를 관찰하였다. 10% 농도의 용출액에서 배양된 유치 줄기세포의 흡광도 값은 RM > EN > EZ 순이었다(p= 0.0439). 그러나 유치 줄기세포는 재료에 관계없이 간엽줄기세포의 표현형을 유지했다. 세 종류의 CSM이 줄기세포 marker를 변형시키진 않았지만, EZ가 RM과 EN보다 세포적합성이 낮음을 알 수 있었다.
Trans Abstract
The purpose of this study was to compare and evaluate the cytotoxicity of 3 calcium silicate-based materials (CSMs) on stem cells from human exfoliated deciduous teeth (SHEDs). The powder of Retro MTA® (RM), EZ-SealTM (EZ) and ENDOCEM Zr® (EN) was eluted with SHED culture media and then filtered. The SHEDs were cultured in the presence of the various concentrations of the eluate. To investigate the effect of the 3 CSMs on SHED proliferation, the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was performed. Flow cytometry analysis was also performed to identify any changes in the cellular phenotype. The absorbance values of the SHEDs cultured in the eluate of samples at a 10% concentration showed the following relation: RM > EN > EZ (p = 0.0439). However, the SHEDs maintained their mesenchymal phenotype regardless of product exposure. Although the 3 CSMs did not alter the SHED stem cell markers, EZ may be a less cytocompatible than RM and EN.
Ⅰ. Introduction
Asymptomatic primary molars with pulp exposed by caries are commonly treated by pulpotomy[1]. The pulpotomy procedure is performed to remove inflammatory and degenerative coronal pulp tissue and to induce healing in the normal radicular pulp tissue[2]. At this time, biocompatible materials should be used to form a protective layer above the exposed vital pulp[3,4].
Mineral trioxide aggregate (MTA) is a Portland cementbased calcium silicate hydraulic cement introduced in the early 1990s[5]. This material has excellent biocompatibility and sealing ability and can induce hard tissue formation. Therefore, it is used in various clinical procedures, such as pulp capping, pulpotomy and apexification[6-9]. Many previous studies have reported successful results for pulpotomy with MTA[1,10,11]. Among the different kinds of MTA products, the most widely used product in the world is ProRoot MTA® (PR; Dentsply Tulsa Dental, Tulsa, OK, USA), and many previous studies on the cytotoxicity of PR have demonstrated its biocompatibility[6]. However, PR has drawbacks, including a long setting time (3 hours), tooth discoloration and a high price[5,12].
To complement the disadvantages of the original MTA, various MTA-like materials, i.e., new calcium silicate-based materials (CSMs), have recently been developed. In other countries, CSMs such as MTA-Angelus® (Angelus, Londrina, PR, Brazil), BiodentineTM (Septodont, Saint Mauré des Fosses, France), Bioaggregate® (Innovative BioCeramix Inc., Vancouver, Canada) and MM-MTATM (Micromega, Besançon, France) have been come onto market[13]. In Korea, Retro MTA® (RM; BioMTA, Seoul, Korea), EZ-SealTM (EZ; EZEKIEL, Taean, Korea) and ENDOCEM Zr® (EN; Maruchi, Wonju, Korea) have been developed and used in clinical practice. Several studies on the biocompatibility of RM and EN have been reported[13-15]. However, EZ is a recently developed CSM and has been used in clinical practice without experimental evidence of its biocompatibility. Moreover, no studies have evaluated the biocompatibility of EZ compared with RM and EN.
The purpose of this study was to compare and evaluate the cytotoxicity of 3 CSMs with stem cells from human exfoliated deciduous teeth (SHEDs).
Ⅱ. Materials and Methods
In this in vitro study, 3 representative CSMs that are commercially available and clinically used in Korea were analyzed: RM, EZ and EN. These materials are composed of powder formulations and can be mixed with normal saline or distilled water, thus enabling the cytotoxicity of the powder alone to be evaluated. IRM® (Dentsply), which was considered a negative control, was excluded because it is composed of a powder and liquid, and the cytotoxicity of both the powder and liquid could not be assessed.
This study was approved by the Institutional Review Board of Korea in accordance with the research policy (W1713/001-001).
1. Cell culture
The SHEDs were purchased from Cell Engineering For Origin Co., Ltd. (CEFO, Seoul, Korea) and were confirmed to possess the characteristics described by Lee et al .[16] in 2011. The frozen SHEDs were rapidly thawed at room temperature and then cultured in a 5% CO2 atmosphere at 37°C using CEFOgroTM DPSC medium (CEFO).
2. Preparation of CSM eluates
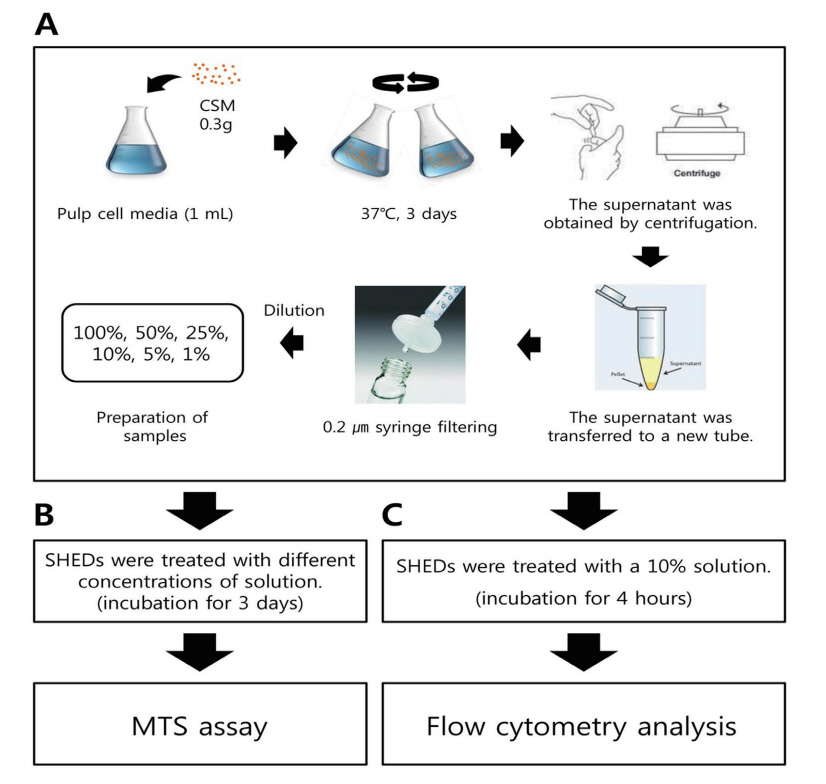
The RM, EZ and EN (0.3 g) powder was sufficiently reacted with 1 mL of SHED medium before elution and filtration (Fig. 1A). First, the flask was shaken sufficiently well to allow the CSM to react; then, the reaction was allowed to continue for 3 days at 37°C. After the reaction was completed, the supernatant was obtained by centrifugation and transferred to a new tube. The supernatant was filtered using a 0.2 μm pore size syringe and diluted to 100%, 50%, 25%, 10%, 5% and 1% for sample preparation. Cell culture medium without CSM powder was used as a control.
3. MTS assay
After trypsinization, cells were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 hours, the solution obtained by eluting the CSM was diluted to 100%, 50%, 25%, 10%, 5% and 1% and used to replace the medium in each well. After the SHEDs were cultured in various concentrations of medium containing CSM for 3 days, the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was performed using an EZ-Cytox Cell Assay Kit (Daeil Lab Service Co., Seoul, Korea) for 1 hour at 37°C (Fig. 1B). The absorbance of EZ-Cytox at 490 nm was measured with a microplate reader (SpectraMAX® M3; Molecular Devices, Sunnyvale, CA, USA).
4. Flow cytometry analysis
The eluate was diluted with SHED culture medium to a concentration of 10% and used to treat the cells for 4 hours in culture. After the cells were collected, they were washed with cold phosphate-buffered saline (PBS) and then washed once more with PBA solution prepared by dissolving 2% bovine serum albumin (BSA) in PBS. In accordance with the study by Lee et al .[16], primary antibodies against CD29 (Cat.#SC-9970, Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD34 (Cat.#CBL486, Millipore, Billerica, MA, USA), CD105 (Cat.#MAB2152, Millipore), CD146 (Cat.#MAB16985, Millipore) and STRO-1 (Cat.#MAB1038, R&D, Minneapolis, MN, USA), which are known to be expressed in stem cells and SHEDs, were diluted with PBA and incubated with the cells for 1 hour under refrigerated conditions. Subsequently, the cells were washed twice with PBA and reacted for 30 minutes or longer with a secondary antibody (mouse IgG-FITC, Cat.#315-095-003, Jackson Immuno Research, USA) diluted in PBA. The cells were then washed twice with PBS and fixed with 3.7% formaldehyde (Cat.#F8775, Sigma, USA). Prepared cell samples were analyzed for cell surface markers using a FACS CaliburTM flow cytometer (BD Science) and CellQuestTM Pro program (Fig. 1C).
5. Statistical analysis
All experiments were performed at least in triplicate, and data from the MTS assay were analyzed using GraphPad PRISM® statistical analysis software (GraphPad, San Diego, CA). Significant differences among groups were identified by the Kruskal-Wallis test. The above analyses were performed using STATA 14.0 (StataCorp, College Station, Texas, USA).
Ⅲ. Results
1. MTS assay
The MTS assay was performed to test the cytotoxic effect of the 3 CSMs on SHED proliferation. The overall absorbance values of the SHEDs cultured in the eluates of various concentrations showed the following relation: EN > EZ > RM. Moreover, in the presence of the 3 CSMs eluates, diluted 1 : 1 (at 100% concentration), cell proliferation was almost fully inhibited, with lower rates than that of the control group; however, no significant differences in proliferation were found among the three groups (Fig. 2). Accordingly, the cytotoxicity of the 3 materials to SHEDs at each concentration was compared, and a significant difference was observed only at a concentration of 10% (p= 0.0439). At this concentration, the cytotoxicities of the eluates were ordered as follows: EZ > EN > RM > control (Fig. 3).

Effects of 3 calcium silicate-based materials on cytotoxicity as measured by MTS assay.
Kruskal-Wallis test (* : p < 0.05)
OD = Optical Density, EN = ENDOCEM Zr®, EZ = EZ-SealTM, RM = Retro MTA®.
The data are presented as mean and standard error (SE) values.
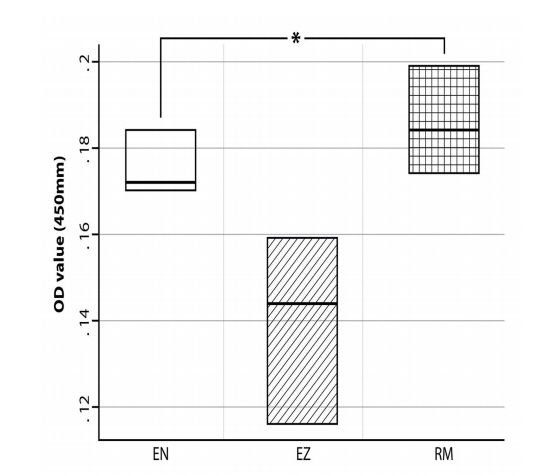
Effects of 3 calcium silicate-based materials at 10% concentrations on cytotoxicity, as measured by MTS assay. Statistically significant difference was observed among the 3 materials.
Kruskal-Wallis test (* : p < 0.05)
OD = Optical Density, EN = ENDOCEM Zr®, EZ = EZ-SealTM, RM = Retro MTA®.
The data are presented as mean and standard error (SE) values.
2. Flow cytometry analysis
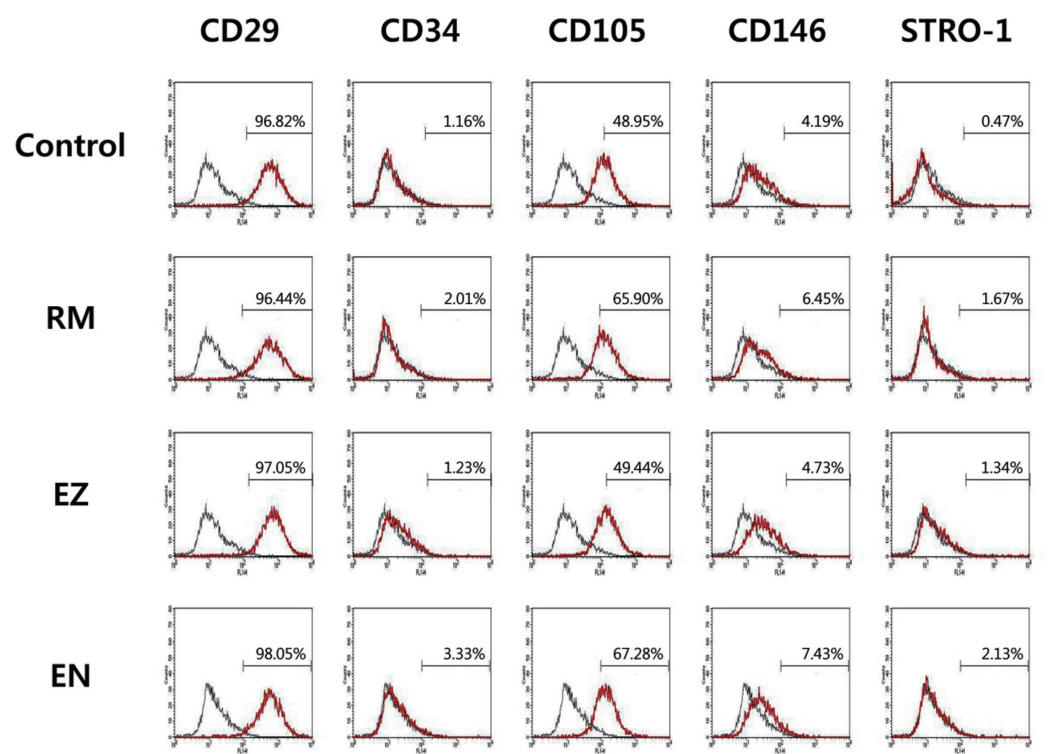
In accordance with the study by Lee et al .[16], the SHEDs expressed CD29, CD105, CD146, STRO-1 and low levels of CD34. In this study, flow cytometry analysis was performed to determine the phenotypical changes caused by exposure to various CSMs. The results of the analysis of cell surface protein markers are as follows (Fig. 4). Overall, CD34 expression was negative, but CD34 expression was lower than 8% in the EZ group. In contrast, CD29 expression was positive in more than 85% of cells, and CD105 expression was positive in more than 75%. CD146 expression was positive in approximately 20%. However, overall STRO-1 expression was less than 5% or almost not expressed. In general, the expression of these markers was similar regardless of product exposure.
Ⅳ. Discussion
The purpose of this study was to analyze the cytotoxicity of RM, EZ and EN powder, which are CSMs used in pulpotomy for primary teeth. RM consists of a hydraulic calcium-zirconia complex and is characterized by a short initial setting time of 150 seconds. It consists of calcium carbonate (60 - 80 wt%), silicon dioxide (5 - 15 wt%), aluminum oxide (5 - 10 wt%) and a calcium-zirconia complex (20 - 30 wt%)[17]. EZ consists of zirconium dioxide (23.5 wt%), calcium carbonate (48 wt%), silicon dioxide (17 wt%), calcium sulfate hemihydrate (10 wt%) and sodium carboxymethyl cellulose (1.5 wt%). It has a setting time of approximately 70 minutes[18]. EN is an MTA-derived pozzolan cement with an initial setting time of 4 minutes[19]. It consists of zirconium dioxide (43 - 46 wt%), calcium oxide (27 - 37 wt%), silicon dioxide (7 - 11 wt%), aluminum oxide (3 - 5 wt%), magnesium oxide and ferric oxide (3 - 5 wt%)[17].
Original MTA is composed of Portland cement with 20% bismuth oxide as a radiopacifier[20]. However, the 3 tested CSMs have a commonality in that they include zirconium oxide instead of bismuth oxide, and this zirconium oxide has many advantages. Min et al .[21] and Kim et al .[22] showed that bismuth oxide exhibits toxicity to human dental pulp cells and periodontal ligament cells in vitro. In contrast, Li et al .[23] reported that ZrO2 positively affects the viability of MG63 cells in vitro, as shown by MTT assay. In addition, bismuth oxide causes tooth discoloration[5], while zirconium oxide causes less discoloration. The degree of discoloration depends on the amount of metals, such as bismuth, iron, aluminum and magnesium oxide[17]. The composition of each of the abovementioned materials shows that EZ does not contain heavy metal components. The manufacturer claims that RM also contains no heavy metals, but aluminum is included. In addition, EN contains not only aluminum but also iron and magnesium oxide. Therefore, all 3 products are said to cause no discoloration, but further and longer-term studies are needed to examine their potential for tooth discoloration. In addition, zirconium oxide shortens the setting time of the material because zirconium accelerates the hydration of Portland cement[5,23].
To determine the cytotoxicity of the CSM eluates to SHEDs, MTS assay was performed, and the results are expressed as absorbance (optical density, OD) values. Herein, the OD value represents the amount of light absorbed by the cells and is a measure of the number of cells. The number of surviving cells was small in the presence of the 100% eluates, and the effect on the 3 materials on cell proliferation decreased with decreasing concentration, indicating that the cytotoxicity is dosedependent. However, the absorbance values for the 3 CSMs did not show significant differences by the Kruskal-Wallis test, except at a concentration of 10% (p= 0.008); cell proliferation was affected by the 10% CSM eluates. Accordingly, this concentration was selected for evaluating cytotoxicity in the subsequent flow cytometry analysis.
The flow cytometry analysis showed that the SHEDs treated with the 10% CSM eluates and those in the control group retained characteristics of mesenchymal stem cells, as indicated by negative CD34 (hematopoietic marker) and positive expression of CD29, CD105, CD146 and STRO-1 (mesenchymal markers). Thus, as the expression patterns of cell surface markers were the same for both the treated and untreated SHEDs, the CSM eluates did not affect the SHED characteristics.
While there have been several previous studies on the cytotoxicity and biocompatibility of PR, RM and EN, the results have been controversial. According to a study by Lee et al .[13] that assessed the pulpal response after pulpotomy in dog’s teeth, the pulpal response to RM was slightly lower than that to PR; however, the difference was not significant, indicating that RM can be used as an alternative to PR with similar biocompatibility. Chung et al .[14] reported that RM has similar biocompatibility to PR for human dental pulp cells and that EN shows temporary initial cytotoxicity. These researchers speculated that EN has a higher concentration of aluminum than PR[5], which could cause cytotoxicity; however, they suggested that this assumption cannot be confirmed because RM also includes Al2O3. In the current study, although EZ does not contain aluminum or heavy metals that could affect cytotoxicity, the absorbance values in this group were lower than those in the EN group. Therefore, the assumption that aluminum affects the cytotoxicity can be rejected. Kim et al .[15] have shown that EN has similar biocompatibility to PR in experiments (in vitro and in vivo) on human primary dental pulp cells, indicating that it can be used as an alternative for PR. In contrast, Lee et al .[24] have shown that while RM and EN reduce the viability of human primary dental pulp cells, EN supported better cell viability with a lower alkalinity than other materials.
To evaluate the cytotoxicity of the dental materials, a contact experiment between cells and the experimental materials was performed. The method of contact include direct contact, indirect contact and eluate contact[25]. CSMs consist mainly of tricalcium and dicalcium silicate that reacts with water and finally hardens[26]. During the hydration process, calcium hydroxide (CH) is produced, which increases the pH. It has been reported that the pH increases from 10.2 to 12.5 over 3 hours after MTA manipulation and that the continuous release of CH results in the maintenance of a high pH for 78 days[27,28]. It is significant that this study excluded the influence of the pH increase in the setting process of the materials by evaluating the cytotoxicity of the un-hydrated powder only, in the pre-polymerization stage, by the elution method. Additionally, there have been many reports on the cytotoxicity and biocompatibility of various types of MTA on animal and human cells. However, most of the studies are on pulp-derived cells and periodontal ligament cells, as mentioned above. Therefore, this study is significant as the first study to evaluate the cytotoxicity of RM, EZ and EN with SHEDs.
In vitro cytotoxicity experiments are the most fundamental experiments that must be performed before the clinical use of a material, but there is a limitation in that these experiments do not reflect factors of the clinical environment, such as tissue inflammation. Despite these limitations, flow cytometry analysis showed that the 3 CSMs did not alter the SHED phenotype. However, the MTS assay showed that EZ exerted the greatest cytotoxicity among the 3 materials. Further in vivo/clinical studies including a large number of subjects and a long-term evaluation of the 3 CSMs are needed.
Ⅴ. Conclusion
Based on this study’s results, the following conclusions can be made: The cytotoxicity of EZ was the greatest among the 3 materials. However, EZ, RM and EN did not alter the stem cell markers of SHEDs. Thus, EZ may be a less cytocompatible than RM and EN.