|
 |
| J Korean Acad Pediatr Dent > Volume 46(2); 2019 > Article |
|
ņ┤łļĪØ
ņØ┤ ņŚ░ĻĄ¼ņØś ļ¬®ņĀüņØĆ ĻĖĆļØ╝ņŖż ņĢäņØ┤ņśżļģĖļ©Ė ņŗ£ļ®śĒŖĖņØś Ēæ£ļ®┤ ļ│┤ĒśĖĻ░Ć ļ»ĖņäĖĻ▓ĮļÅäņÖĆ ļ¦łļ¬©ņĀĆĒĢŁņä▒ņŚÉ ļ»Ėņ╣śļŖö ĒÜ©Ļ│╝ļź╝ ņĢīņĢäļ│┤Ļ│Āņ×É ĒĢśļŖö Ļ▓āņØ┤ļŗż. ĻĖĆļØ╝ņŖż ņĢäņØ┤ņśżļģĖļ©ĖņÖĆ ļĀłņ¦äĻ░ĢĒÖöĒśĢ ĻĖĆļØ╝ņŖż ņĢäņØ┤ņśżļģĖļ©Ėļź╝ ņé¼ņÜ®ĒĢśļĀż Ļ░üĻ░ü 60Ļ░£ņØś ņŗ£ĒÄĖņØä ņĀ£ņ×æĒĢśņśĆļŗż. Ļ░ü ņŗ£ĒÄĖņØä Ēæ£ļ®┤ ļ│┤ĒśĖļź╝ ņŗ£Ē¢ēĒĢśņ¦Ć ņĢŖņØĆ Ļ▓ĮņÜ░, ļéśļģĖĒĢäļ¤¼Ļ░Ć ĒĢ©ņ£ĀļÉ£ Ēæ£ļ®┤ ļ│┤ĒśĖņ×¼, ĒĢäļ¤¼Ļ░Ć ĒĢ©ņ£ĀļÉśņ¢┤ ņ׳ņ¦Ć ņĢŖņØĆ Ēæ£ļ®┤ ļ│┤ĒśĖņ×¼ņŚÉ ļö░ļØ╝ 20Ļ░£ņö® ļéśļłäņŚłļŗż. 37┬░C ņ”ØļźśņłśņŚÉ 24ņŗ£Ļ░ä ļ│┤Ļ┤ĆĒĢ£ Ēøä Ļ░ü ĻĄ░ļŗ╣ 10Ļ░£ņØś ņŗ£ĒÄĖņØĆ ļ╣äņ╗żņŖż ļ»ĖņäĖĻ▓ĮļÅäļź╝ ņĖĪņĀĢĒĢśņśĆĻ│Ā, 10Ļ░£ņØś ņŗ£ĒÄĖņØĆ ļ¦łļ¬© ņŗ£ĒŚśņØä ņŗ£Ē¢ēĒĢ£ Ēøä ļ¦łļ¬©ļÉ£ Ļ╣ŖņØ┤ļź╝ ņĖĪņĀĢĒĢśņśĆļŗż. Ēæ£ļ®┤ ļ│┤ĒśĖļź╝ ņŗ£Ē¢ēĒĢ£ ĻĄ░ļōżļ│┤ļŗż Ēæ£ļ®┤ ļ│┤ĒśĖļź╝ ņŗ£Ē¢ēĒĢśņ¦Ć ņĢŖņØĆ ĻĄ░ļōżņØ┤ ļåÆņØĆ Ēæ£ļ®┤Ļ▓ĮļÅäļź╝ ļ│┤ņśĆļŗż. ĻĖĆļØ╝ņŖżņĢäņØ┤ņśżļģĖļ©ĖņÖĆ ļĀłņ¦äĻ░ĢĒÖöĒśĢ ĻĖĆļØ╝ņŖżņĢäņØ┤ņśżļģĖļ©Ė ļ¬©ļæÉņŚÉņä£ Ēæ£ļ®┤ ļ│┤ĒśĖļź╝ ņŗ£Ē¢ēĒĢ£ Ļ▓ĮņÜ░ņŚÉ ļ¦łļ¬©ņĀĆĒĢŁņä▒ņØ┤ ļŹö ņ”ØĻ░ĆĒĢśņśĆņ¦Ćļ¦ī ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ņĢäļŗłņŚłļŗż. ļéśļģĖĒĢäļ¤¼ņØś ņ£Āļ¼┤ļŖö ļ¦łļ¬©ļÅäņŚÉ ņ£ĀņØśĒĢ£ ņśüĒ¢źņØä ļ»Ėņ╣śņ¦Ć ļ¬╗ĒĢśņśĆļŗż.
Abstract
The purpose of this study was to investigate the effect of adding a protective coating on the microhardness and wear resistance of glass ionomer cements (GICs).
Specimens were prepared from GIC and resin-modified GIC (RMGI), and divided into 3 groups based on surface protection: (1) no coating (NC), (2) Equia coat coating (EC), and (3) un-filled adhesive coating (AD). All specimens were then placed in distilled water for 24 h. Surface hardness (n = 10) was evaluated on a Vickers hardness testing machine. Wear resistance (n = 10) was evaluated after subjecting the specimen to thermocycling for 10,000 cycles using a chewing simulator. Data were analyzed using a one-way ANOVA and the Kruskal-Wallis test.
Surface hardness was highest in the NC groups, followed by the EC and AD groups. The wear depth of GI + NC was significantly higher than that of all RMGI groups. EC did not significantly lower the wear depth compared to AD.
Based on these results, it was concluded that although EC does not increase the surface microhardness of GIC, it can increase the wear resistance.
Glass ionomer cement (GIC) is a tooth-colored restorative material that releases fluoride and chemically bonds to tooth structure[1]. This material has a coefficient of thermal expansion similar to that of a tooth, and is widely used in pediatric dentistry without a bonding agent[2-4].
However, some properties of GIC may limit its application[5]. GIC has low surface hardness, strength, and wear resistance compared to other restorative materials such as amalgam and composite resins[6]. The long setting reaction time and susceptibility to moisture during setting reaction restricts its widespread clinical use[7].
In order to overcome these drawbacks, resin-modified GI (RMGI) was introduced at the end of the 1980s with improved mechanical properties as well as operability and aesthetics[8]. During the early stage of setting, photopolymerization of the resin component occurs first and is followed by chemical acid group reaction of the glass ionomer component, resulting in a fully matured double polymerized product[9].
During the initial stage, which occurs within first 10 minutes after mixing, GIC is sensitive to water uptake. The second stage, slower acid-base reaction, lasts 24 hours and is susceptible to dehydration[10]. Therefore, it is recommended to protect the surface of GIC during the first 24 hours to avoid a decrease in the mechanical properties of the GIC application[5].
In order to protect the restorative material from water contamination, immediate application of a surface coating agent is recommended[11]. These include solvent based and lightcured bonding resins, petroleum jelly, or fluoride varnish[5]. Recently, a new generation of coating for GIC known as the Equia coat, which is a self-adhesive resin containing nanofillers, has been introduced. The manufacturer claims that Equia coat protects the restoration from wear and dissolution, increases the surface hardness, and mechanical stress is dispersed due to the evenly dispersed nanofillers and tough coating layer.
However, a few studies have compared the surface protective coating effect of the Equia coat with non-filled resin adhesive. And none of these studies have evaluated the wear resistance of GI and RMGI under thermocycling conditions. Therefore, this study was carried out to evaluate whether nanofilled resinbased coatings increase the microhardness and wear resistance of GI and RMGI when stored in water for 24 hours, compared to coating with non-filler adhesives and no surface coating.
The materials used in this study are listed in Table 1. 60 discs with a diameter of 9.0 mm and a thickness of 2.0 mm were fabricated for each specimen. Fuji IX GP EXTRA (GI) and Fuji IX LC (RMGI) were used according to the manufacturerŌĆÖs instructions and mixed for 10.0 seconds using Rotomix (3 M ESPE, Seefeld, Germany). After injection into the mold, the samples were covered with transparent matrix strips (Matrix-Strips, Orbis, M├╝nster, Germany) and a transparent glass slab was placed over them. GI was allowed to set for 150.0 seconds while RMGI was light cured for 20.0 seconds using a light-emitting diode (LED) with a standard power mode (1000.0 mW/cm2).
The specimens were then removed from the mold and the surfaces were ground using #600 grit silicon carbide paper. The specimens were then randomly grouped into 3 batches (20 in each batch for each material). The first batch (NC) was considered as the control and was not subjected to any further treatment. A thin layer of Equia coat was applied to the surface of the specimens in the second batch (EC) using a micro brush and light cured for 20 seconds. An unfilled adhesive, Scotchbond Multi-Purpose (3M Dental Products, St Paul, MN., USA), was similarly applied over the surfaces of the specimens in the third batch (AD) with a micro brush and light cured for 20.0 seconds. All the specimens were labeled, stored, and tested after being immersed for 24 h in distilled water at 37.0Ōäā.
10 specimens per group were subjected to a hardness test for 15.0 s at 300.0 g load using a Vickers microhardness test machine (Mitutoyo, Kawasaki, Japan). The Vickers machine comprises a diamond shaped indenter with a square base (Vickers pyramid) and with an opening angle of 136.0┬░ which is pressed vertically on to the surface of the objects being tested. Each specimen was subjected to three indentations and the average values were calculated.
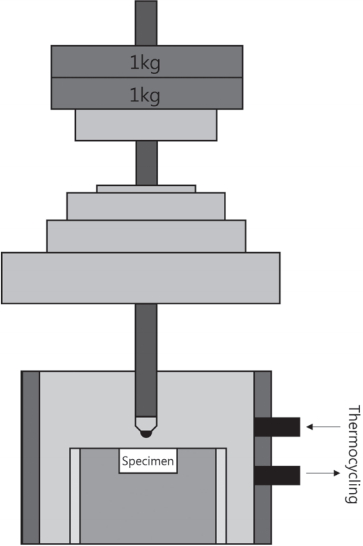
10 specimens per group were subjected to a wear test. The wear test was conducted using a masticatory simulator (Chewing simulator SC-4.8; SD Mechatronik, Feldkirchen-Westerhan, Germany) that has 8 chambers simulating the vertical and horizontal movements simultaneously in the thermocycling condition (Fig. 1)[12,13]. Each of the chambers consists of an upper metal antagonist and a lower plastic sample holder in which the specimen can be embedded. For fixation, the specimens were embedded in acrylic resin in the lower holder. A 5.0 mm vertical movement and a 3.0 mm horizontal movement were reproduced with a vertical load of 2.0 kg for 10,000 cycles at 1.7 Hz in the presence of simultaneous thermal stress (Table 2).
The wear depths (╬╝m) of specimens were determined using a micrometer (Mitutoyo, Kawasaki, Japan).
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) software. The Shapiro-Wilk normality test was used to ensure that the normality assumption of all the wear data was met. Mean values and standard deviation of Vickers hardness were calculated and analyzed using a oneway ANOVA. Post hoc analyses among group means were conducted using a Tukey test. The wear test was analyzed with the Kruskal-Wallis test and bonferroniŌĆÖs post hoc test.
The mean and standard deviation values of the different samples are shown in Tables 5 and 6. The GI + NC group had the highest wear rate while RMGI + EC had the lowest wear rate. All GI groups had a higher wear rate than that of the RMGI groups.
A significantly higher amount of wear was noted with GI + NC compared to RMGI + NC (p= 0.030), RMGI + EC (p= 0.000), and RMGI + AD (p= 0.010). No significant difference was observed within the GI and RMGI groups. GI + AD showed a significantly higher wear rate than that oh RMGI + EC (p= 0.012). The wear rate of GI + EC was not significantly different from that of RMGI + EC, which had with the lowest wear rate (p= 0.286).
The setting reaction of GI is an acid-base reaction, which is caused by the interaction of the poly acid liquid with the glass powder. The acid attacks the glass network and releases cations such as Al3+ and Ca2+ or Sr2+. Within the first 10 minutes after mixing, calcium polyacrylate, which is vulnerable to hydrolysis, is formed. This matrix is subsequently converted to a more stable form (aluminum polyacrylate) over the first 24 hours[14-16]. Therefore, the cement surface must be protected from water contamination during the setting reaction to prevent the dissolution of metal cations. Reduced mechanical properties and increased surface corrosion and wear tendency has been reported in water contaminated (during early setting) GIC restorations [17].
Resin-modified GICs were developed in the 1980s to enhance the weak physical properties of conventional GICs. With the addition of 2-hydroxyethylmethacrylate (HEMA), a hydrophilic resin monomer, and a photoinitiator[18], RMGI had a higher compressive strength when compared with conventional GI during the initial setting stage (within the first 24 h)[10]. However, since RMGI still has the properties of conventional GIC, it is important to prevent early water contamination and dehydration. Miyazaki et al .[19] claimed that the surface of RMGI should be protected from water contamination for at least 1 hour after cement mixing. In this study, compared to non-surface protected specimens, both GI and RMGI specimens with surface protection showed significantly lower surface hardness. This result is opposed to the manufacturerŌĆÖs claim that the nanofilled coating increases surface hardness of the restoration. The surface hardness of the surface protection materials was lower than that of the restorations. Faraji et al .[20] reported that conventional GI with nanofilled coating exhibited lower Vickers hardness than GI without coating. This may be due to the thickness of the coating itself, since the coating was applied in accordance with the manufacturerŌĆÖs instructions (light cured without additional air drying). In general, protective coatings do not have the required mechanical properties of a suitable restorative material.
The manufacturers of Equia coat report that it contains nanofillers. Fillers are the strongest content in resin components that are added to strengthen the composite resin and decrease the percentage of resin monomer. Kim et al .[21], noted that increasing the filler content enhanced the mechanical properties of the restorative material. Shinkai et al .[22], reported that restoration containing small sized fillers exhibited better wear resistance compared with that of restorations containing large fillers. Therefore, the higher surface microhardness in specimens coated with the Equia coat in this study is thought to be due to the filler content compared to the fillerfree adhesive.
All GI groups had lower Vickers hardness number than that of the RMGI groups. Addition of the resin component in RMGI not only decreases initial hardening time and handling difficulties, but also increases physical strength of the cement[23].
Wear resistance is an important property for all restorative dental materials. This is the ability of the restoration to withstand the grinding force of the opposing tooth and food, while maintaining its function. Despite some beneficial properties, GICs have been proven unsuitable for stress-bearing areas due to their poor wear resistance[24,25]. The use of EC has been recommended by its manufacturer as it strongly bonds to GI and improves abrasion resistance. The chewing simulator is a two-body wear test machine and the main wear mechanism acting in this study was abrasion in combination with surface fatigue[26].
In both GI and RMGI, surface protection increased wear resistance compared to NC, but the difference was not statistically significant. This seemed to be due to the protection from water contamination during the first 24 hours of initial hardening. Hotta et al .[27] reported that the use of light-polymerized agents can restrict water movement across the cement surface. In previous studies, the surface protection significantly increased wear resistance, but in this study, wear resistance between coated groups and uncoated groups did not show statistically significant differences. This may be due to the small sample size and large deviation of results of this study.
In this study, EC increased the wear resistance compared to AD in both GI and RMGI. This may be due to the microfiller contained in the EC. However, the difference was not statistically significant. Therefore, it would be expected that the effect of the nanofiller is less than that of water contamination prevention which is increased the wear resistance.
The RMGI has improved physical properties because of its resin monomer, and shows excellent initial strength. Croll and Nicholson[23] reported that fracture toughness, fracture resistance, and resistance to wear are all improved in the resinmodified glass ionomer. However, Lohbauer[10] claimed that RMGI are more prone to abrasive wear due to a weak fillermatrix coupling. In this study, uncoated GI showed the lowest wear resistance. However, no significant difference was observed between all RMGI groups and GI when surface protection was applied to GI, regardless of the coating type. These results suggest that GI is more sensitive to early water contamination than RMGI, and therefore it is thought to have a greater effect on coatings.
There are a few limitations of this study. The hardness of only the outermost surface protection material was assessed and it does not reflect that of the actual restorative material. Further, this study was conducted in the laboratory without recreating the complete oral environment. Additionally, the specimens were stored in water for only 24 hours, and hence the longterm effect on the surface coating could not be evaluated.
Application of surface protection did not have a major influence on the hardness of the two types of GI cements. However, the wear resistance of GI increased following the use of surface protection and was similar to that of RMGI. The presence of nanofillers did not significantly affect wear resistance.
Table┬Ā1.
Materials used in this study
Table┬Ā2.
The experimental conditions of chewing simulator
Table┬Ā3.
Mean vickers hardness number and standard deviation of materials
| Mean Vickers Hardness Number ┬▒ SD | ||
|---|---|---|
| GI | RMGI | |
| NC | 21.35 ┬▒ 1.93 | 51.57 ┬▒ 3.03 |
| EC | 16.12 ┬▒ 2.42 | 32.37 ┬▒ 3.11 |
| AD | 12.95 ┬▒ 4.20 | 26.32 ┬▒ 3.85 |
Table┬Ā4.
Correlation between values of vickers hardness number
| GI + NC | GI + EC | GI + AD | RMGI + NC | RMGI + EC | RMGI + AD | |
|---|---|---|---|---|---|---|
| GI + NC | ||||||
| GI + EC | 0.007 | |||||
| GI + AD | 0.000 | 0.245 | ||||
| RMGI + NC | 0.000 | 0.000 | 0.000 | |||
| RMGI + EC | 0.000 | 0.000 | 0.000 | 0.000 | ||
| RMGI + AD | 0.012 | 0.000 | 0.000 | 0.000 | 0.001 |
Table┬Ā5.
Mean and standard deviation of materials wear depth
| Mean wear depth ┬▒ SD | ||
|---|---|---|
| GI | RMGI | |
| NC | 55.25 ┬▒ 31.67 | 19.30 ┬▒ 10.17 |
| EC | 26.35 ┬▒ 16.87 | 11.25 ┬▒ 7.15 |
| AD | 30.50 ┬▒ 10.09 | 16.30 ┬▒ 9.86 |
References
1. Mount GJ, Makinson OF : Glass-ionomer restorative cements: clinical implications of the setting reaction. Oper Dent, 7:134-141, 1982.

2. Catelan A, Briso AL, Sundfeld RH, Dos Santos PH : Effect of artificial aging on the roughness and microhardness of sealed composites. J Esthet Restor Dent, 22:324-330, 2010.


3. Marquezan M, Osorio R, Ciamponi AL, Toledano M : Resistance to degradation of bonded restorations to simulated caries-affected primary dentin. Am J Dent, 23:47-52, 2010.

4. Francci C, Deaton TG, Bawden JW, et al. : Fluoride release from restorative materials and its effects on dentin demineralization. J Dent Res, 78:1647-1654, 1999.


5. Bonif├Īcio CC, Werner A, Kleverlaan CJ : Coating glass-ionomer cements with a nanofilled resin. Acta Odontol Scand, 70:471-477, 2012.


6. van Duinen RN, Kleverlaan CJ, Feilzer AJ, et al. : Early and long-term wear of ŌĆśfast-setŌĆÖ conventional glass-ionomer cements. Dent Mater, 21:716-720, 2005.


7. de Gee AJ, van Duinen RN, Werner A, Davidson CL : Early and long-term wear of conventional and resin-modified glass ionomers. J Dent Res. 1619, 1996.
8. Lacy AM, Young DA : Modern concepts and materials for the pediatric dentist. Pediatr Dent, 18:469-478, 1996.

9. Kim GS, Kim YK : The effect of various surface coatings on microleakage and microhardness of light-cured glass ionomer restoration. J Korean Acad Pediatr Dent, 24:495-510, 1997.
10. Lohbauer U : Dental Glass Ionomer Cements as Permanent Filling Materials? - Properties, Limitations Future Trends. Materials (Basel), 3:76-96, 2010.

11. Kishore G, Sai-Sankar AJ, Sai-krishna VS, et al. : Comparative Evaluation of Fluoride Releasing Ability of Various Restorative Materials after the Application of Surface Coating Agents - An In-vitro Study. J Clin Diagn Res. 41, 2016.

12. Jung YS, Lee JW, Huh JB, et al. : A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. J Adv Prosthodont, 2:111-115, 2010.



13. Park JH, Park S, Lim HP, et al. : Antagonist wear of three CAD/CAM anatomic contour zirconia ceramics. J Prosthet Dent, 111:20-29, 2014.


14. Leirskar J, Nordb├Ė H, Mount GJ, Ngo H : The influence of resin coating on the shear punch strength of a high strength auto-cure glass ionomer. Dent Mater, 19:87-91, 2003.


16. Mitra SB, Kedrowski BL : Long-term mechanical properties of glass ionomers. Dent Mater, 10:78-82, 1994.


17. Gemalmaz D, Yoruc B, Ozcan M, Alkumru HN : Effect of early water contact on solubility of glass ionomer luting cements. J Prosthet Dent, 80:474-478, 1998.


18. Uno S, Finger WJ, Fritz U : Long-term mechanical characteristics of resin-modified glass ionomer restorative materials. Dent Mater, 12:64-69, 1996.


19. Miyazaki M, Moore BK, Onose H : Effect of surface coatings on flexural properties of glass ionomers. Eur J Oral Sci, 104:600-604, 1996.


20. Faraji F, Heshmat H, Banava S : Effect of protective coating on microhardness of a new glass ionomer cement: Nanofilled coating versus unfilled resin. J Conserv Dent, 20:260-263, 2017.



21. Kim KH, Ong JL, Okuno O : The effect of filler loading and morphology on the mechanical properties of contemporary composites. J Prosthet Dent, 87:642-649, 2002.


22. Shinkai K, Taira Y, Suzuki M, et al. : Effect of filler size and filler loading on wear of experimental flowable resin composites. J Appl Oral Sci. 20160652, 2018.


23. Croll TP, Nicholson JW : Glass ionomer cements in pediatric dentistry: review of the literature. Pediatr Dent, 24:423-429, 2002.

24. Frencken JE : The ART approach using glass-ionomers in relation to global oral health care. Dent Mater, 26:1-6, 2010.


25. Zhao J, Weng Y, Xie D : A novel high-wear-resistant glassionomer cement for class I and class II restorations. Eur J Oral Sci, 117:86-89, 2009.


- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 2,741 View
- 150 Download
- Related articles
-
Effect of Calcium Hydroxide on the Microhardness of Root Dentin of Primary Tooth2013 August;40(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print