치아우식 예방을 위한 치아미백기의 활용 : 광역동 치료로서의 접근
Application of Teeth Whitening LED for Prevention of Dental Caries : Antimicrobial Photodynamic Therapy Approach
Article information
Abstract
이 연구의 목적은 악궁 전반적으로 조사가 가능한 미백 LED를 이용하여 우식원성 Streptococcus mutans에 대한 광역동 치료의 효과를 알아보기 위한 in vitro 연구이다.
S. mutans를 각각 두가지 다른 단계의 상태로 배양하였다. Planktonic 상태와 Hydroxyapatite disk와 CDC biofilm reactor를 5일간 사용하여 동적인 바이오필름 상태를 형성하였다. 20 μM 로 희석한 erythrosine을 5분간 전처리한후 가정용 미백기와 진료실 미백기를 사용하여 각 15분씩 광조사를 시행하였다. 실험 종료 후 각 실험군의 Colony Forming Unit를 측정하였으며 공초점 레이저 현미경를 이용하여 각 조건에 따른 광역동 치료 효과를 비교하였다.
실험 결과 S. mutans에 대한 aPDT효과는 control군과 비교시에 각각 플랑크토닉상태와 동적인 바이오필름 상태에서 모두 통계적으로 유의한 광역동 치료 효과가 나타났다(각각 p = 0.001, p = 0.002). 하지만 동적인 바이오필름 상태에서는 플랑크토닉 상태보다 광역동치료에 내성을 지녀 항미생물 효과가 떨어지는 양상이 관찰되었다. 진료실용 미백 LED와 가정용 미백LED는 유사한 항미생물 효과를 나타냈다.
구강악궁 전반적 조사가 가능한 미백용 LED는 우식의 원인이 되는 S. mutans 바이오필름에 유의한 광역동 치료의 효과를 나타냈으며, 이러한 결과는 치아 우식 예방으로의 광역동 치료의 임상적 적용 가능성을 제시한다.
Trans Abstract
The present study is aimed to assess the effect of antimicrobial photodynamic therapy (aPDT) on Streptococcus mutans biofilm through teeth whitening light emitting diode (LED).
Planktonic and dynamic biofilm state cultures of S. mutans were used. Erythrosine 20 μM/L was used as the photosensitizer. Irradiation was performed by exposing cultures to clinic and homecare whitening LEDs for 15 minutes. The viability was measured through Colony Forming Unit counts and confocal laser scanning microscopy.
aPDT using whitening LEDs and erythrosine significantly decreased the CFU count of S. mutans compared to that in the control group. Dynamic biofilm group showed more resistant features to aPDT compared with planktonic state. Clinic and homecare whitening LED device showed similar antimicrobial effect.
The whitening LED, which could irradiate the entire oral arch, showed a significant photodynamic effect on cariogenic S. mutans biofilm. aPDT mediated by erythrosine and LEDs used for teeth whitening exhibited promising antimicrobial activity.
Ⅰ. Introduction
Control and prevention of oral biofilms are a global issue in dentistry. Despite the widespread education and increased awareness on the management of oral biofilms, dental caries remains a costly burden[1]. The importance of prevention of dental caries a subject of great importance.
Streptococcus mutans causes the development of dental caries, and a reduction in the abundance of S. mutans in the oral cavity can help reduce or eliminate dental caries[2]. The biofilms that cause caries are attached to the enamel surface of the teeth.
Mechanical removal is the most common method of controlling biofilms. However, it depends on patient compliance. Antiseptic mouthwashes can be used to reduce the overall load of oral flora, but prolonged use leads to various complications such as alterations in taste, oral mucosa desquamation, and black hairy tongue[3].
Antimicrobial photodynamic therapy (aPDT) is an established treatment in the medical field for localized cancer and dermatological conditions[4]. The significant advantage and strength of aPDT are that it can be used as a targeted therapy. Selective targeting and reduced toxicity without the development of drug resistance allows the repeated use of this treatment[5]. aPDT involves interaction between a photosensitizer, which absorbs light, and a light source to activate the oxygen species. Photoactivated reactive oxygen species (singlet oxygen and toxic radicals) begin oxidizing organic molecules, resulting in localized photodamage and death of microorganisms[6]. In dentistry, aPDT has been proven to be very effective in the reduction of microbial load in the oral biofilms on teeth and soft tissue surfaces[7-9]. Therefore, aPDT can be a useful tool for controlling biofilms and preventing the risk of caries.
Previous studies have verified the effectiveness of aPDT by studying various light sources such as laser, halogen light, and light emitting diodes (LED). Although the light sources used in previous studies were all effective at reducing the microbial load[10,11], they cannot cover the entire oral cavity and dental arch due to the limited range of area covered by light radiation. Hence, there is the need for a light delivery system that can compensate for the disadvantages of the light sources previously used.
Whitening LED is designed to deliver light effectively and evenly throughout the dental arch and oral cavity. There are no previous reports of whitening devices being used as aPDT light delivery systems. Since oral biofilm management is mainly performed at home, this study was performed using both a homecare whitening device and a whitening device used in clinics. PDT with a useful light delivery source covering the entire dental arch might be a new option for the control of caries. The objective of this in vitro study was to evaluate the antimicrobial effect of aPDT using whitening LED device on planktonic and biofilm cultures of S. mutans .
Ⅱ. Materials and Methods
1. Photosensitizer and light source
In this study, erythrosine (Sigma-Aldrich, St. Louis, MO, USA) was chosen as a photosensitizer. The erythrosine concentration was 20 μM/L and it was prepared in phosphate buffered saline (PBS, pH 7.4), sterilized by filtration, covered in an aluminum foil, and stored at -20°C[11-13].
Two types of LEDs for teeth whitening were used as light sources: Clinic whitening LED unit (YS-TW-A, Yunsheng Medical Instrument, Guangdong, China) and whitening LED for homecare (Dr. Smile, Rosapacific, Seoul, South Korea). The whitening LEDs used in the clinic had wavelengths in the range of 450 to 490 nm and a power density of 44 mW/cm2, whereas whitening LEDs for home-use had wavelengths in the range of 420 to 540 nm and a power density 0.02 mW/cm2. The power output of each curing unit was checked using a radiometer (LED Radiometer; Three H, Guangzhou city, China). Light irradiation was performed 2.0 cm above hydroxyapatite disk.
2. Bacterial strain
Streptococcus mutans ATCC 25175 strain was used in this study. The organism was inoculated into a brain heart infusion broth (BHI; Becton, Dickinson and Company, Sparks, MD, USA) and cultured at 37°C for 18 hours in a 5% CO2 incubator. A spectrophotometer (Smart Plus 2700; Young-woo inst., Seoul, Korea) was used to measure the turbidity of the bacterial suspension. The number of bacteria was adjusted using a standard curve.
(1) Planktonic culture
100 μL of a bacterial suspension at a final concentration of 107 CFU/mL was added to each well of a 96-well cell culture plate (SPL Life Sciences, Pocheon-si, Gyeonggi-do, Korea). For the erythrosine supplement group, 100 μL of erythrosine was added, whereas 100 μL of PBS was added to the control groups[14,15].
(2) Biofilm culture
Biofilms were cultured in a Center for Disease Control and Prevention (CDC) biofilm reactor (Fig. 1A). Hydroxyapatite (HA) disk was used to reproduce the surface of enamel for developing biofilms. Each disk was mounted onto a rod that could hold 3 disks. The back side of the fixed HA disk coupons was coated with silicone (Examixfine injection type, GC Corporation, Tokyo, Japan) so that biofilm could be formed only on the top side of the coupon (Fig. 1B). After ethylene oxide (EO) gas sterilization, 8 disk rods were fixed inside the CDC biofilm reactor, and 300 mL BHI broth and 100 mL of the bacterial suspension adjusted to 1 × 105 CFU/mL were added. The medium was cultured for 24 hours without vortexing and medium feeding. After 24 hours, the inflow and outflow of the BHI medium were controlled using a peristaltic pump (Jenie Well, Seoul, South Korea) at a rate of 5.6 mL/min for 72 hours, and the agitator was operated at 50 rpm for vortexing.
3. Photodynamic therapy
Each HA disk coupon was gently washed outside on a sterile plate with 2 mL PBS twice to remove loosely attached bacteria on the HA disk. The experimental groups were classified as shown in Table 1. Erythrosine was applied to the groups Ⅱ, Ⅴ, and Ⅵ for 5 minutes. Groups Ⅲ and Ⅴ were irradiated with home care whitening LEDs and groups Ⅳ and Ⅵ were irradiated with clinic whitening LEDs for 15 minutes.
4. Colony forming unit counting
After treatment, each HA disk coupon was moved into a 24 well plate with silicone removed. HA disks were transferred to 2 mL PBS and sonicated with an ultrasonic device (VC 100; Sonics & Materials Inc., Danbury, CT, USA) twice for 10 seconds to dissipate the biofilm. Each sample was diluted with PBS. Fifty microliters of the diluted suspension were poured and spread on blood agar plates in duplicate (Hanil-KOMED, Seongnam, Gyeonggi-do, Korea). The blood agar plates were incubated in 5% CO₂ at 37°C for 72 hours, and total CFUs were counted by an automatic colony counter program (Flash & Go colony counter, IUL, S.A.). All the procedures were repeated independently twice on different days.
5. Cell viability test by confocal laser scanning microscopy (CLSM)
For visualization of the viability, each specimen of biofilm coupon was evaluated by CLSM. Each specimen was washed twice with 400 μL PBS and stained with 200 μL of LIVE/DEAD® BacLight™ Bacterial Viability Kit solution (Molecular Probes, Inc., Eugene, OR, USA) containing SYTO-9 (green, viable cells) and propidium iodide (red, dead cells). Staining was performed inside a dark box, at room temperature for 15 minutes, according to the manufacturer’s instructions. After 15 minutes, biofilms were rinsed gently with 100 μL of distilled water. HA disks were moved on to a glass slide and were examined with a LEICA TCS SP8 confocal microscope at 10× magnification, using an excitation laser (argon gas laser, 488 nm) and HyD detector (485/540 nm for detection of SYTO9 and 601/670 nm for detecting propidium iodide). The collected images were processed and analyzed by an image-processing program (Leica application Suite X, Switzerland).
6. Statistical analysis
Analysis of the aPDT effect was performed in duplicate, and all procedures were independently repeated on different days. Data were analyzed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Kruskal-Wallis test was used for evaluating CFU/mL data. Each group was compared by Mann-Whitney U test, and the p value was measured by the Bonferroni correction for post-hoc analysis. The level of significance was set at p < 0.003.
Ⅲ. Results
1. CFU values
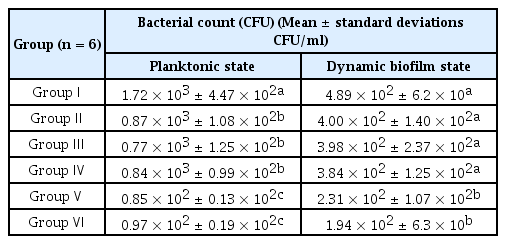
The mean and standard deviations of the results in the planktonic state and biofilm state are specified in Table 2 and Fig. 3 and 4.
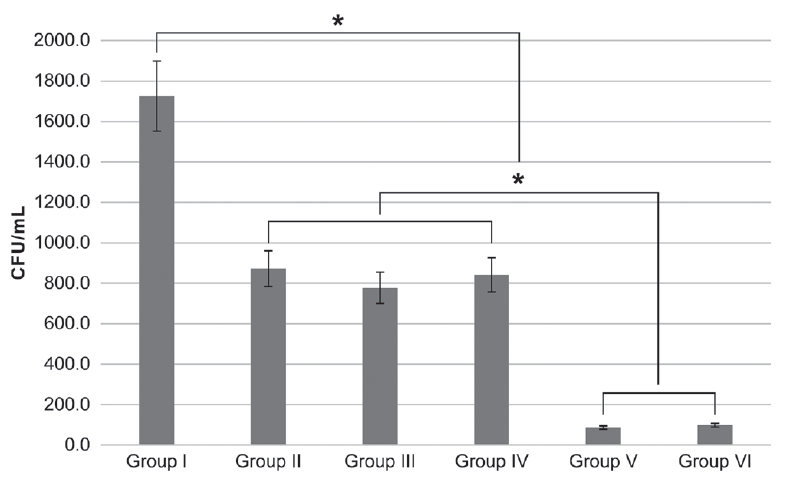
Streptococcus mutans planktonic colony forming unit count after different photodynamic treatments. Bonferonni test as post-hoc test (* : p < 0.003).
(1) Group Ⅰ: Control (2) Group Ⅱ: Photosensitizer only (3) Group Ⅲ: Homecare whitening LED (4) Group Ⅳ: Clinic whitening LED (5) Group Ⅴ: Photosensitizer + Homecare whitening LED (6) Group Ⅵ: Photosensitizer + Clinic whitening LED.
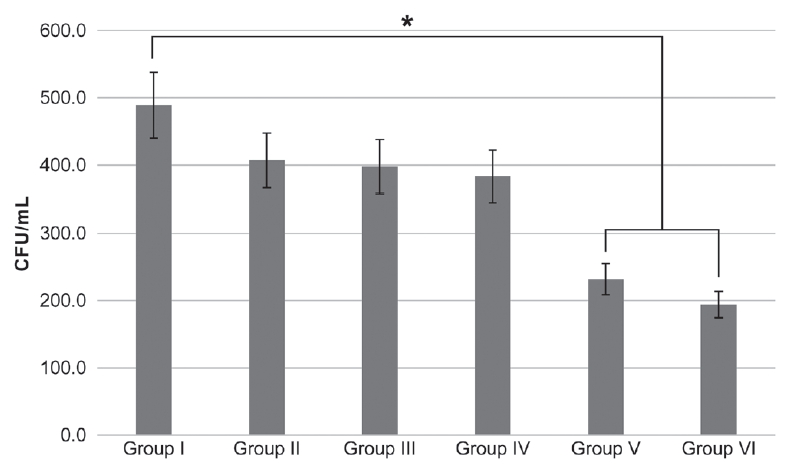
Streptococcus mutans dynamic biofilm colony forming unit count after different photodynamic treatments. Bonferroni test as post-hoc test (* : p < 0.003).
(1) Group Ⅰ: Control (2) Group Ⅱ: Photosensitizer only (3) Group Ⅲ: Homecare whitening LED (4) Group Ⅳ: Clinic whitening LED (5) Group Ⅴ: Photosensitizer + Homecare whitening LED (6) Group Ⅵ: Photosensitizer + Clinic whitening LED.
Compared with those in the control group, CFU counts decreased in both planktonic and biofilm states in aPDT-treated groups V and VI (p = 0.001, p = 0.002 each). The groups of erythrosine with clinic whitening LED groups (group IV) showed 95% and 61% reduction in CFU counts of planktonic state and biofilm state respectively (Fig. 4)
In the planktonic state, the use of erythrosine (group Ⅱ) and LED light irradiation (group Ⅲ, Ⅳ) caused decrease of CFU count (p = 0.002, Fig. 3).
2. Confocal laser scanning microscopy
The effects of aPDT on biofilms of S. mutans were visualized with CLSM. Viability was assessed by staining and comparing live cells (green) and dead cells (red). As shown in Fig. 5, majority of the cells showed green fluorescence in the control group (group Ⅰ), and the aPDT-treated groups (groups V and VI) showed substantially enhanced red fluorescence, which increased with increasing irradiation intensity.
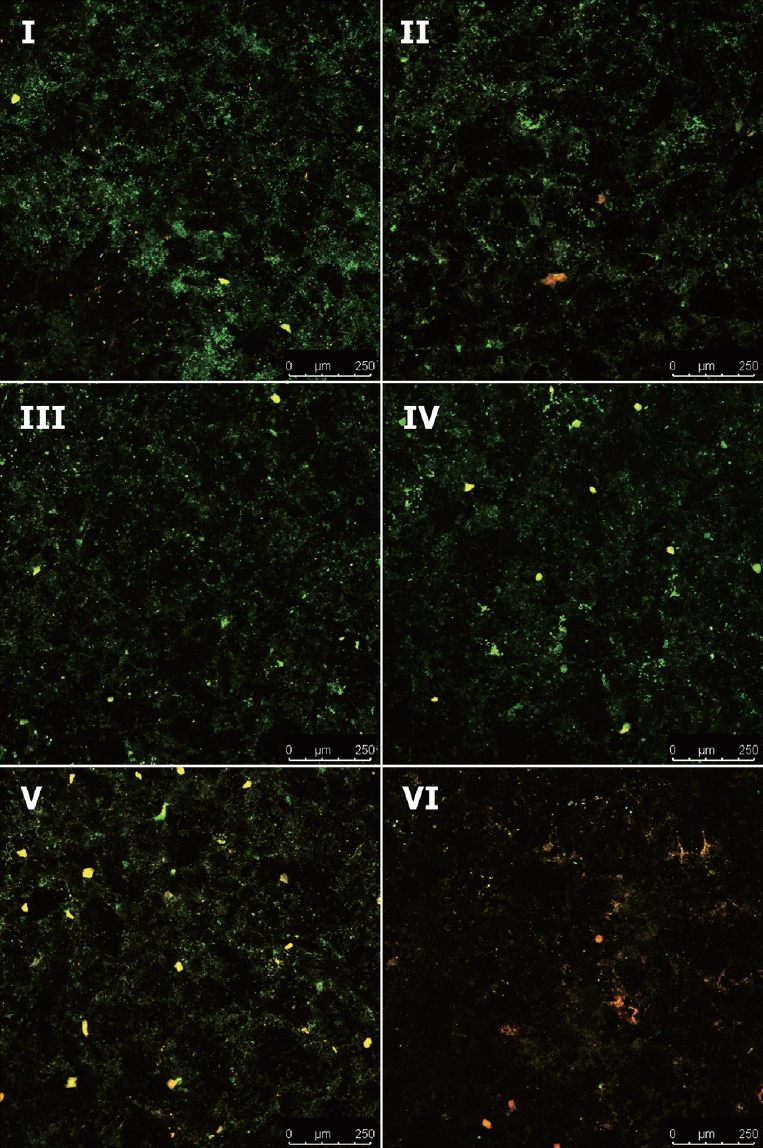
Confocal Laser Scanning Microscopy images of S. mutans dynamic biofilms. Green indicates viable cells, red indicates dead cells.
(1) Group Ⅰ: Control (2) Group Ⅱ: Photosensitizer only (3) Group Ⅲ: Homecare whitening LED (4) Group Ⅳ: Clinic whitening LED (5) Group Ⅴ: Photosensitizer + Homecare whitening LED (6) Group Ⅵ: Photosensitizer + Clinic whitening LED.
Ⅳ. Discussion
aPDT consists of 3 crucial components, namely light source, a nontoxic photosensitizer, and oxygen. In this study, the photosensitizer was erythrosine. Erythrosine is frequently used in dental practice at concentrations of 20 mM as a plaque disclosing agents. The clinical concentrations is approximately 1000 times higher than that used in the present study. Safety of erythrosine mediated photodynamic therapy has been proven in previous studies[16,17].
Various light sources and their energy level have been tested for aPDT effects. Kim et al.[11] showed effective aPDT effects on S. mutans regardless of the light sources (halogen light, LED, laser) with same energy of 18000 mW/cm2 applied. However these conventional light delivery systems have limitation’s of not being able to cover the entire oral cavity. Despite various aPDT studies have been conducted in the past, there are no precise protocol in the prevention or treatment of dental caries. The absence of a protocol for aPDT in dentistry is mainly due to the localized radiation radius of the light source leading a inefficient procedure. To overcome this limitation, whitening LED was used as a light delivery source. Whitening LED has a broad diameter of irradiance that can effectively distribute light throughout the dental arch.
In this study, the total irradiation time was set as 15 minutes. The energy of photodynamic therapy with erythrosine, which was most effective with halogen light, was observed to be above 18 J[12,13]. Since the output of the home whitening LED was 0.02mW/cm2, the power density of 18 J requires 15 minutes of light irradiation. Additionally the application time of the homecare and clinical whitening LED devices was 15 minutes according to the manufacturer’s instruction. The experiment was designed on the assumption that the time spent on whitening could be spent on photodynamic therapy.
In a previous study that focused on the effect of whitening LED and the increase of intrapulpal temperature[18], 5.5°C was considered the critical level of tolerance for intrapulpal temperature. There was an increase in intra-pulpal temperature with all whitening LED tested. Among the results, the blue LEDs which had a energy density of 1248 J caused 2°C increase of intrapulpal temperature. Clinic whitening LED used in this study had an energy density of 39 J, which can be interpreted to have limited effect on the pulp.
In this study, both clinic whitening LED, and homecare LED exhibited a promising antimicrobial effect on the planktonic and biofilm states of S. mutans . The difference in the power density between homecare whitening LED and clinic whitening LED devices did not cause a difference in their antibacterial activity. The reason for the similar results between the low output homecare whitening LED (0.02mW/cm2) and high output of clinical whitening LED (44mW/cm2) can be explained by wavelengths of photosensitizers. The wavelength of the homecare whitening LED (420 - 540 nm) was similar to the erythrosine wavelength (440 - 570nm)[19]. If the wavelength of a light source matches that of a photosensitizer, it can produce an effective photodynamic therapeutic effect, but even if the wavelengths do not match, blue LED alone produces antibiotic effects. According to de Souse et al.[20] violet-blue light can serve as an adjunct prophylactic treatment for reducing S. mutans biofilm formation and enamel mineral loss.
In our study, aPDT groups showed 95% reduction in planktonic states, whereas in dynamic biofilm state, the reduction rate ranged from 53% to 61%. It is well known that bacteria are considerably more resistant in biofilms than in planktonic cultures[11,21,22]. Although aPDT was less effective in the treatment of bacteria with dense biofilms than in planktonic culture, the difference was less than twofold. Whereas studies on antibiotics and subgingival plaques have reported aPDT to be approximately 250-fold less effective in biofilm conditions compared to planktonic cultures[21].
Due to the susceptible property of planktonic cultures, reduction in viability was observed in not only aPDT group but also in erythrosine only treated groups (II) and LED only treated groups (III, IV). This is consistent with previous reports that erythrosine alone had an inhibition effect on the growth of S. mutans [23,24]. And the results of this study coincide with previous studies which demonstrated the antimicrobial activity against S. mutans with blue light alone[20,25-27].
There are several limitations in this study. An in vitro study was conducted, which could not accurately reproduce the biofilm in the oral cavity, and a single S. mutans strain was used to produce biofilms. Future in vivo studies using whitening LED involving actual biofilms in the oral cavity are needed. Another limitation is the 15 minute irradiation time used in photodynamic treatment. Patients compliance with this 15 minute irradiation can be a challeng. Moreover, it is necessary to consider the change of light source effect by the saliva flow in oral cavity, when applying the whitening LED device for 15 minutes.
An indicator of a clinically significant effect of photodynamic therapy is an 3 log reduction. The 50% antimicrobial effect on biofilms is not clinically acceptable. It is due to the low energy of selected whitening LED and low concentration of photosensitizers. Further research designed to reduce irradiation time by increasing the light intensity or increasing the concentration of erythrosine is required.
Ⅴ. Conclusion
Present study confirmed the positive effects of aPDT and whitening LED on the reduction of S. mutans . Whitening LED may be useful in the prevention of dental caries by managing dental biofilms at different stages of their development. Rather than a narrow, localized irradiation on each teeth, the use of a whitening LED that allows full arch irradiation may provide clinicians and patients with a more straight forward approach to manage biofilms.