1. Aldred MJ, Crawford PJ : Variable expression in Amelogenesis imperfecta with taurodontism.
J Oral Pathol, 17:327-333, 1988.


2. Collins MA, Mauriello SM, Tyndall DA, Wright JT : Dental anomalies associated with amelogenesis imperfecta: a radiographic assessment.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 88:358-364, 1999.


3. Ooya K, Nalbandian J, Noikura T : Autosomal recessive rough hypoplastic amelogenesis imperfecta. A case report with clinical, light microscopic, radiographic, and electron microscopic observations.
Oral Surg Oral Med Oral Pathol, 65:449-458, 1988.


4. Peters E, Cohen M, Altini M : Rough hypoplastic amelogenesis imperfecta with follicular hyperplasia.
Oral Surg Oral Med Oral Pathol, 74:87-92, 1992.


5. Parry DA, Mighell AJ, Inglehearn CF,
et al. : Mutations in CNNM4 cause Jalili syndrome, consisting of autosomalrecessive cone-rod dystrophy and amelogenesis imperfecta.
Am J Hum Genet, 84:266-273, 2009.



6. Ababneh FK, AlSwaid A, AlBalwi MA,
et al. : Hereditary deletion of the entire FAM20C gene in a patient with Raine syndrome.
Am J Med Genet A, 161:3155-3160, 2013.

7. Wright JT, Johnson LB, Fine JD : Development defects of enamel in humans with hereditary epidermolysis bullosa.
Arch Oral Biol, 38:945-955, 1993.


8. Wright JT, Hong SP, Luder HU,
et al. : DLX3 c.561_562delCT mutation causes attenuated phenotype of tricho-dentoosseous syndrome.
Am J Med Genet A, 146:343-349, 2008.

9. O’Sullivan J, Bitu CC, Dixon MJ,
et al. : Whole-Exome sequencing identifies FAM20A mutations as a cause of amelogenesis imperfecta and gingival hyperplasia syndrome.
Am J Hum Genet, 88:616-620, 2011.



10. MacGibbon D : Generalized enamel hypoplasia and renal dysfunction.
Aust Dent J, 17:61-63, 1972.


11. Kala Vani SV, Varsha M, Sankar YU : Enamel renal syndrome: a rare case report.
J Indian Soc Pedod Prev Dent, 30:169-172, 2012.


12. Witkop CJ : Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification.
J Oral Pathol, 17:547-553, 1988.


13. Normand Tranchade I, Bonarek H, Nancy J,
et al. : Amelogenesis imperfecta and nephrocalcinosis: a new case of this rare syndrome.
J Clin Pediatr Dent, 27:171-175, 2003.


14. Seow WK : Clinical diagnosis and management strategies of amelogenesis imperfectavariants.
Pediatr Dent, 15:384-393, 1993.

15. Feller L, Jadwat Y, Raubenheimer EJ,
et al. : Enamel dysplasia with odontogenic fibroma-like hamartomas: review of the literature and report of a case.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 101:620-624, 2006.


16. Kantaputra PN, Bongkochwilawan C, Chaisrisookumporn YN,
et al. : Periodontal disease and FAM20A mutations.
J Hum Genet, 62:679-686, 2017.



17. Paula LM, Melo NS, Acevedo AC,
et al. : Case report of a rare syndrome associating amelogenesis imperfecta and nephrocalcinosis in a consanguineous family.
Arch Oral Biol, 50:237-242, 2005.


18. Martelli-Junior H, Bonan PR, Coletta RD,
et al. : Case reports of a new syndrome associating gingival fibromatosis and dental abnormalities in a consanguineous family.
J Periodontol, 79:1287-1296, 2008.


19. Yonemochi H, Noda T, Saku T : Pericoronal hamartomatous lesions in the opercula of teeth delayed in eruption: an immunohistochemical study of the extracellular matrix.
J Oral Pathol Med, 27:441-452, 1998.


20. Cho YA, Yoon HJ, Hong SD,
et al. : Multiple calcifying hyperplastic dental follicles: comparison with hyperplastic dental follicles.
J Oral Pathol Med, 40:243-249, 2011.


21. O’Connell S, Davies J, Smallridge J, Vaidyanathan M : Amelogenesis imperfecta associated with dental follicular-like hamartomas and generalised gingival enlargement.
Eur Arch Paediatr Dent, 15:361-368, 2014.



22. Dure-Molla M, Quentric M, Bloch-Zupan A,
et al. : Pathognomonic oral profile of Enamel Renal Syndrome (ERS) caused by recessive FAM20A mutations.
Orphanet J Rare Dis, 9:84, 2014.



23. Wang SK, Aref P, Hu JC,
et al. : FAM20A mutations can cause enamel-renal syndrome (ERS).
PLoS Genet, 9:e1003302. 2013.



24. Jaureguiberry G, Dure-Molla M, Kleta R,
et al. : Nephrocalcinosis (enamel renal syndrome) caused by autosomal recessive FAM20A mutations.
Nephron Physiol, 122:1-6, 2012.


25. Cho SH, Seymen F, Kim JW,
et al. : Novel FAM20A mutations in hypoplastic amelogenesis imperfecta.
Hum Mutat, 33:91-94, 2012.


26. Proctor , Kumar RN, Porter S,
et al. : Oral and dental aspects of chronic renal failure.
J Dent Res, 84:199-208, 2005.




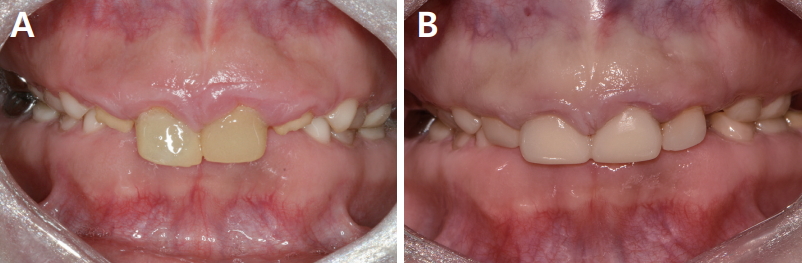










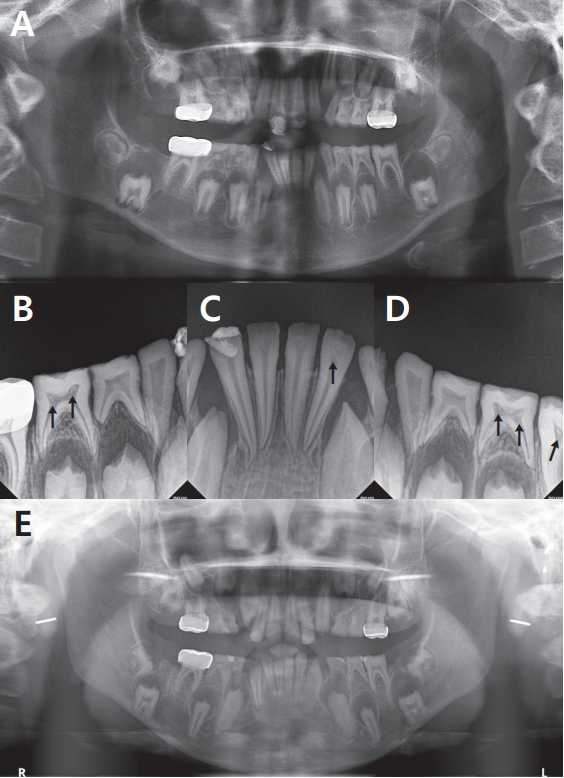
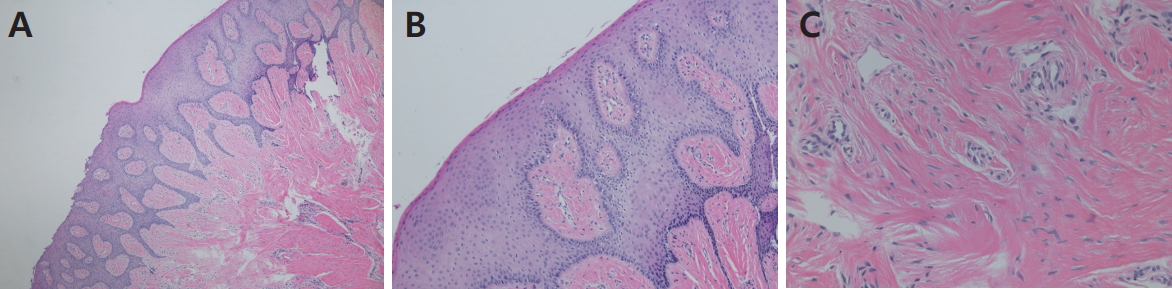
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print