어린이의 유구치에서 우상치의 유병률 분석
Analysis of the Prevalence of Taurodont Deciduous Molars in Children
Article information
Abstract
우상치는 수직적으로 긴 치수강과 근첨부에 위치하는 치수저를 특징으로 가지는 발육성 형태 이상이다. 이 연구의 목적은 어린이의 유구치에서 우상치의 유병률과 특징을 알아보기 위한 것이다.
이 연구는 2005년부터 2018년까지 연세대학교 치과대학병원 소아치과에 내원하여 파노라마 방사선 사진을 촬영한 만 5세에서 10세 사이의 환자 2,473명을 대상으로 시행되었으며 총 19,784개의 유구치가 평가되었다. 우상치의 유병률은 약 5.7%었고 남아에서 더 호발하였다. 또한 상악에서 6.3%, 하악에서 93.6%로 하악에서 현저히 높은 유병률을 보였다. 좌우에 따른 유병률의 차이는 나타나지 않았다. 우상치의 세부 유형으로 Daito의 방법을 적용하여 Hypertaurodontism, Mesotaurodontism, Hypotaurodontism으로 분류하였으며 각 유병률은 11.9%, 78.2%, 7.8%로 Mesotaurodontism이 가장 높은 유병률을 보였다.
Trans Abstract
Taurodontism is an anomaly characterized by a long and broad pulpal cavity and consumed apical location of the furcation area. This study aimed to determine the prevalence of taurodontism in deciduous molars based on digital panoramic radiographs of children. The study was performed on a sample of panoramic radiographs taken from 2,473 Korean children who visited the department of pediatric dentistry, Yonsei University Dental Hospital between Nov. 2005 and Mar. 2018. Taurodontism was mainly categorized by Daito’s method. Using panoramic radiographs, taurodontism was also categorized into mesotaurodontism, hypotaurodontism, and hypertaurodontism. Mesotaurodontism was the most prevalent type. A total of 2,473 panoramic radiographs were evaluated. The prevalence of taurodontism was 5.7% in general, 51.5% in the left quadrant, 48.5% in the right quadrant, 6.3% in the maxilla, and 93.7% in the mandible. The distribution of taurodontism stratified by gender showed a higher prevalence in males. This is a comprehensive study on the prevalence of taurodontism in children using the largest sample size to date.
Ⅰ. Introduction
Taurodontism is a developmental tooth anomaly characterized by an elongated pulp chamber below the level of the alveolar crest and the cementoenamel junction (CEJ) and apical displacement of the furcation of the roots, resulting in a shortened root[1]. The name ‘taurodont’ originated from its resemblance to the teeth of ungulates, especially bull’s teeth, which have a root branch located in the most inferior part of the tooth when observed with the naked eye. ‘Tauro’ means bull in Latin and ‘dont’ means teeth in Greek[2].
A taurodont tooth has no constriction of the cervical CEJ, which is presumably because Hertwig’s epithelial root sheath developed without invagination at the designated time. However, the exact etiology of taurodontism is unclear. The characteristic form of a taurodont tooth is located below the alveolar bone in the gingiva; hence, it is difficult to distinguish it from a normal tooth by just observing the form of its crown with the naked eye during clinical examination of the oral cavity. Therefore, it is mainly diagnosed through features identified on radiographs and divided into subcategories of hypotaurodontism, mesotaurodontism, and hypertaurodontism according to the degree of apical displacement of the pulp chamber floor[3,4].
Taurodont teeth are among the most common abnormal forms of permanent teeth[5], and they are known to have a prevalence of 2.5 - 11.3% depending on the race and diagnostic method[6]. Taurodontism also occurs more in permanent teeth than in deciduous teeth; it can occur in all types of teeth and arches[7]. It can occur in a single arch or both arches. Generally, it affects the mandible more than the maxilla[7].
The development of taurodont teeth is also influenced by systemic disorders, including other dental abnormalities and various syndromes[8]. It is associated with cleft lip and palate[9], Down’s syndrome[10-13], ectodermal dysplasia[14], hypophosphatasia[15], hypodontia[16-18], oligodontia[19], amelogenesis imperfecta[20], and tricho-dento-osseous syndrome[21-23]. Studies have also shown that taurodont teeth are found in 75% of patients with Klinefelter syndrome[13,24,25].
Pediatric dentists observe a variety of dental abnormalities during an examination, and these abnormalities should be considered when planning treatment. The purpose of this study was to investigate the prevalence of taurodontism in deciduous molars, analyze its developmental characteristics, and provide useful information to pediatric dentists.
Ⅱ. Materials and Methods
Approval for the study was obtained from the Institutional Review Board (IRB) of Yonsei University Dental Hospital (IRB No: 2 - 2018 - 0022).
1. Subjects
This study was conducted for patients aged between 5 and 10 years who had undergone panoramic radiography at the department of pediatric dentistry, Yonsei University Dental Hospital between 2005 and 2018. The scope was set as such because patients usually begin undergoing panoramic radiography from age 5, and root absorption of deciduous molars usually begins at an approximate age of 10 years. The study only focused on deciduous teeth whose roots were not absorbed by the furcation. To exclude the local factors affecting the evaluation of dental development, cases with dental lesions such as cysts or odontomas were excluded from the investigation. Teeth with a history of damage, such as tooth fractures, moderate or advanced dental caries affecting the dentin, and pulp treatment, among others, were also excluded because they could cause errors in the ratio measurement. A total of 2,473 patients, 1,234 female, and 1,239 male children, were included in the study.
2. Methods
Daito’s method[26] was applied to the detailed classification of the deciduous taurodont teeth (Fig. 1). It is a useful method for evaluating deciduous molars because it can be used when the root is absorbed or when the root formation is incomplete. Assuming the uppermost part of the crown to be A and the CEJ to be B, the vertical distance between these two points, x, was first measured. Assuming the furcation to be C, the vertical distance, y, between B and C was also measured. The ratio between x and y was used for the classification of taurodont teeth; ratio below 0.3, between 0.3 and 0.5, between 0.5 and 1.0, and above 1.0 were diagnosed as normal, hypotaurodontism, mesotaurodontism, or hypertaurodontism, respectively (Fig. 2).
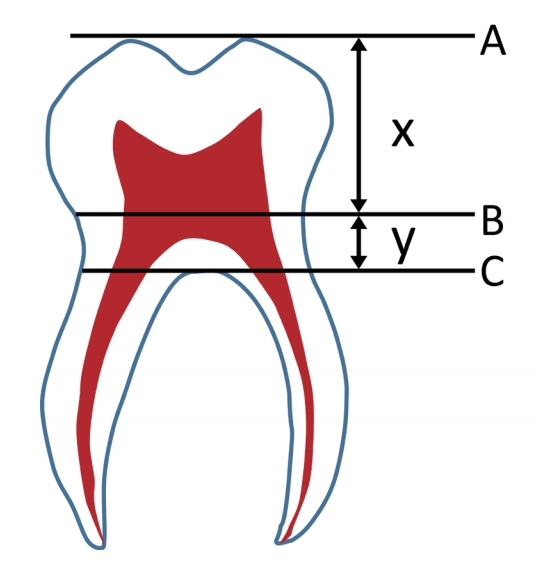
Detection of a taurodontism based on the criteria described by Daito. A : The uppermost part of the crown, B : the cementoenamel junction, C : the furcation, x : the vertical distance between A and B, y : the vertical distance between B and C.
Two well-educated observers performed the radiographic analysis. Before this experiment, the diagnosis of a taurodont tooth using panoramic radiographs was performed twice at two-week intervals to evaluate the inter-observer reliability. Cohen’s kappa coefficient, which represents the inter-observer reliability, was 0.83. In addition, approximately 20 panoramic radiographs were assessed for intra-observer reliability, and Cohen’s kappa coefficient was 0.95.
3. Statistical analysis
Statistical analysis of the data was made using SPSS (version 35.0.0, SPSS, Chicago, IL, USA). The chi-square test was used to compare the prevalence of taurodontism in the maxilla and mandible, and in the right and left quadrants. Also, chi-square test was applied to compare the frequency of taurodontism between male and female patients.
Ⅲ. Results
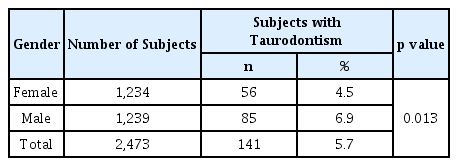
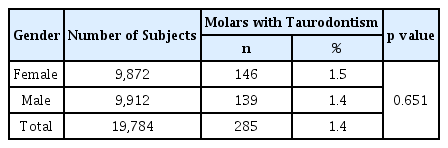
Deciduous taurodont teeth were observed in 141 patients (5.7%) out of the total 2,473 patients included in the study; this represented 285 (1.4%) out of 19,784 teeth.
Of the 141 patients with taurodont tooth 56 were girls and 85 were boys. The prevalence in girls and boys was 4.5% and 6.9%, respectively, which was statistically significant (Table 1, p= 0.013). Of the 285 taurodont teeth, 146 were in girls (1.5%) and 139 were in boys (1.4%); the difference in prevalence was not statistically significant (Table 2). The number of taurodont teeth was slightly higher in girls, but the number of patients who developed the disease was higher in boys. On average, the number of taurodont teeth per person with taurodontism was 2.6 in girls and 1.6 in boys.
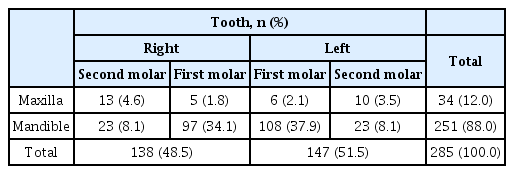
The prevalence of taurodont teeth is demonstrated in Table 3. With 12% in the maxilla and 88% in the mandible, a significantly greater number of taurodont teeth was observed in the mandible than in the maxilla (p= 0.000). The incidence of taurodontism among the mandibular first deciduous molars was very high (71.9%). The prevalences in right and left sides were 48.5% and 51.5%, respectively, and the difference was not statistically significant (p= 0.591).
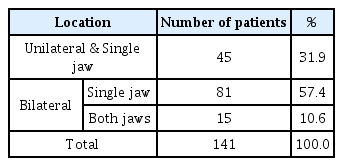
Concerning the location of the taurodont teeth, 57.4% of the cases occurred bilaterally in a single arch and 31.9% occurred unilaterally in a single arch. Patients who had bilateral taurodontism, occurring in both maxillary and mandibular arches, accounted for 10.6% (Table 4).
On stratifying prevalence by classification, mesotaurodontism was the most prevalent (78.2%), followed by hypertaurodontism (11.9%) and hypotaurodontism (9.8%) (Table 5).
Ⅳ. Discussion
Researches have been conducted on the prevalence of taurodont teeth globally, with most reports in the United States showing a prevalence of approximately 2.5 - 3.2% of the population[27], 1.5% in Israel[28], 9.9% in Germany[19], 2.8% in India[29] and 8.6% in Saudi Arabia[30]. According to this study, the prevalence of taurodont teeth in Korean children was 5.7%, which does not deviate significantly from the findings reported for in other races. This difference in prevalence may be attributed to racial differences, or the differences in the evaluation methods.
After the term and concept of taurodontism were first used by Keith[1], Shaw[4] classified it into hypotaurodontism, mesotaurodontism, and hypertaurodontism according to severity. Later, Shifman and Chananel[28] devised a more quantitative classification system based on the ratio of the length from the apex to the roof of the pulp chamber and the length from the pulpal floor to the roof of the pulp chamber, and the length from the CEJ to the pulpal floor; teeth were classified according to their values. This method is currently the most widely used and accepted, but it is difficult to use when the root growth is incomplete or when root absorption is progressing. Therefore, this study used Daito’s method, as mentioned earlier. Since there are several cases of partially absorbed roots of deciduous teeth due to the permanent successors below the teeth, diagnosis should be based on anatomical criteria such as furcation, which can be detected on radiographs. Differences in diagnostic methods contribute to differences in prevalence.
In this study, the prevalences of hypotaurodontism, mesotaurodontism, and hypertaurodontism were 9.8%, 78.2%, and 11.9%, respectively; the prevalence varied across several studies. Similar to this study, mesotaurodontism had the highest prevalence in the study by Munir et al .[31]. On the other hand, Jamshidi et al .[30] showed that the prevalence of hypertaurodontism and mesotaurodontism were 4.8% and 11.1%, respectively, which were lower than that of hypotaurodontism. The study by Bronoosh et al .[32] and Bürklein et al .[33] also reported that hypotaurodontism had the highest prevalence. The limitations of this approach to classification include the difficulty in accurately measuring the CEJ and the inevitable distortion on panoramic radiographs.
The gender-based differences in prevalence have not yet been clarified; however, in this study, the prevalence was slightly higher in boys (6.9%) than in girls (4.5%). Based on the number of teeth, girls had more taurodont teeth, with the average number of taurodont teeth per patient being 2.6 in girls and 1.6 in boys. Therefore, it can be inferred that if a girl develops a malformation, it tends to be more multiplicative. Several studies have shown contradictory results. Bronoosh et al .[32] and Munir et al .[31] showed that women had a higher prevalence than men, but Constant et al .[34], Jafarzadeh et al .[5], and Bürklein et al .[33] did not report a significant difference.
Concerning the prevalence in the maxillary and mandibular arches, a greater number of taurodont teeth were found in the mandible in this study, but some previous studies[35,36] reported that they occur more in the maxilla. However, Bürklein et al .[2] reported that there was no significant difference in prevalence between the maxilla and the mandible. In addition, the prevalence in the left and right arches did not show a significant difference, which was consistent with several previous studies[2,36,37].
It is important to consider these dental abnormalities in a child’s treatment plan. Taurodont teeth, which are easily observed in the clinic, interfere with dental treatment such as pulp treatment. The key differences from ordinary teeth that should be noted include the size and shape of the pulp cavity, the shape of the root canal, apical displacement of the floor of the pulp chamber, and the high likelihood of having accessory canals[4]. Since the sizes of taurodont teeth are usually large, sufficient disinfection with cleaning agents such as sodium hypochlorite (NaOCl) to completely remove the pulp[38] is recommended, and additional methods such as ultrasonic cleaning are needed to ensure that no pulp tissue remains. In addition, when opening the root canal, it is important to be diligent to avoid perforation; because of severe hemorrhaging during the procedure, it is easy to mistakenly establish a perforation even if it is absent. Due to the difficulty of root canal treatment with short roots, a pulpotomy may be considered as the first treatment option as the severity increases.
Taurodont teeth are less stable to use as abutments for prosthetic or orthodontic purposes because their root areas surrounded by the alveolar bone are smaller than those of normal teeth[39]. Yordanova et al .[40] reported that if orthodontic treatment was performed with taurodont teeth, severe side effects could be caused by root absorption and lack of fixation. Given the shape of the short and fine roots of taurodont teeth, the risk of root absorption increases during fixed orthodontic treatment. Therefore, it is necessary to consider root shape parameters such as root length and curvature.
From a periodontal point of view, taurodontism has been reported to have a good prognosis because the probability of inflammation or periodontal pockets in the furcation area when the gingival recession progresses is relatively low[41].
This study was conducted on Koreans, but has limitations in that it was conducted only in children who visited the department of pediatric dentistry, Yonsei University Dental Hospital as well as the proportion of patients who visited for dental treatment would be high. If the number of specimens is high and supplementary assessments, such as periapical radiography and computed tomography (CT), are performed in addition to panoramic radiography, the reliability of research may be further improved.
Taurodont teeth can occur in permanent and deciduous teeth and the maxillary and mandibular arches. Several studies have been conducted on various dental abnormalities, but only a few of them focused on deciduous taurodont teeth. The size of the sample is a major cause of errors; a larger sample size corresponds to higher reliability of the calculated prevalence. Significantly, this study has been conducted on the largest number of deciduous taurodont molars globally. In addition, taurodont teeth are related to several genes and other dental abnormalities, but the exact etiologies are unclear, and further studies are needed.
Ⅴ. Conclusion
This study retrospectively analyzed 2473 panoramic radiographs to determine the prevalence of taurodontism in the deciduous molars of children. The prevalence of taurodontism was 5.7%, and it occurred more frequently in males than in females and the mandible than in the maxilla. Mesotaurodontism was the most common taurodont subtype. Pediatric dentists should carefully examine the oral cavity and radiographs of children to promptly detect and diagnose related diseases.