골격성 Ⅱ급 소아∙청소년의 상기도 공간에 영향을 미치는 요인 : CBCT 연구
Factors Influencing Upper Airway Dimensions in Skeletal Class Ⅱ Children and Adolescents: A CBCT Study
Article information
Abstract
이 연구의 목적은 골격성 2급 부정교합 소아∙청소년의 상기도 공간을 분석하고, 이에 영향을 미치는 요인을 알아보고자 함이다.
총 67명의 골격성 2급 소아∙청소년의 CBCT영상으로 연구를 진행하였다. 상기도 부피와 최소 단면적은 3차원 CBCT 영상을 통해 평가하였으며, 악안면 형태와 골 성숙도는 2차원 두부방사선사진을 통해 평가하였다. 상기도 부피 및 최소 단면적과 다양한 변수들간의 연관성이 분석되었다.
상기도 공간은 최대 성장기 이전의 환자에서 가장 작았으며, 연령과 양의 상관관계를 보였다. 상기도 부피는 전안면 고경 및 연령과, 최소 단면적은 하악 폭경 및 연령과 가장 높은 상관관계를 나타냈다.
골격성 2급 소아∙청소년의 상기도 공간은 연령, 골 성숙도, 세 평면에서의 악안면 형태와 유의한 연관성을 가졌다.
Trans Abstract
The purpose of this study is to investigate factors influencing the upper airway dimensions in skeletal Class Ⅱ children and adolescents.
In total, 67 patients were selected. Airway volume and minimal cross-sectional area were three-dimensionally assessed. Craniofacial morphology and skeletal maturity were assessed on generated two-dimensional cephalograms. The measurements were analyzed using Mann-Whitney test, one-way ANOVA, Pearson’s correlation, and multiple regression analysis.
Upper airway dimensions were significantly smaller in pre-peak stage group, and positively associated with age. Anterior facial height and age were the most relevant factors for airway volume. Mandibular width and age were the most relevant factors for minimal cross-sectional area.
Upper airway dimensions were significantly associated with age, skeletal maturity and craniofacial morphology in all three planes.
Ⅰ. Introduction
The effects of respiratory function on craniofacial growth have been studied for decades. More recently, the association between upper airway configurations, craniofacial development, and sleep-disordered breathing (SDB) has been studied[1,2]. Studies on the association between upper airway dimensions and craniofacial morphology have shown that skeletal Class III patients have a greater airway volume and minimal cross-sectional area than skeletal Class I and Class II patients[3-6].
The association between upper airway space and SDB has been confirmed through various studies. Arens et al .[7] reported that in patients with SDB, the upper airway has a significantly smaller cross-sectional area and volume than that in healthy patients. Kim et al .[8] reported that patients with severe SDB had a smaller upper airway width.
As maxillofacial growth can be influenced by airway dimensions, airway assessment is important in growing patients. There are 2 methods of airway assessment: using lateral cephalograms and cone-beam computed tomography (CBCT) images. A two-dimensional (2D) assessment using skeletal and soft tissue landmarks on a lateral cephalogram lacks accuracy with respect to the actual airway size or structure. Three-dimensionally (3D) reconstructed images from CBCT provide stereoscopic images of the airway and enable measurement of the cross-sectional area and volume of the airway space.
Few studies conducted in Korea have used CBCT to analyze the upper airway space in pediatric patients with skeletal Class II malocclusion. In addition, research on associations between the vertical and transverse craniofacial morphology and the upper airway dimension is limited.
This study aimed to investigate factors influencing the upper airway dimensions in pediatric skeletal Class II patients by assessing the correlations with gender, age, skeletal maturity, and craniofacial dimensions.
Ⅱ. Materials and Methods
This retrospective study was approved by the institutional review board (IRB) of the Wonkwang University (WKDIRB202003-02).
1. Subjects
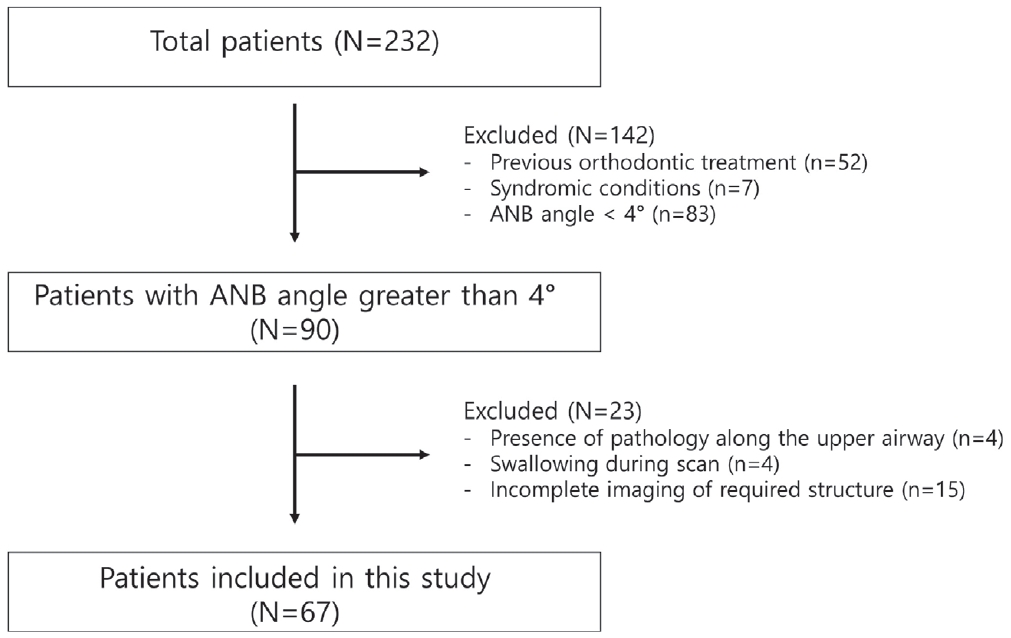
This study retrospectively analyzed 232 patients who had undergone CBCT due to supernumerary teeth, impacted teeth, cystic lesion and orthodontic/surgical reasons at the Wonkwang University Dental Hospital from 2014 to 2019. The inclusion criteria were (1) patients between 8 and 15 years of age, (2) A point-nasion-B point (ANB) angle ≥ 4°, (3) biting in centric occlusion, and (4) CBCT scans with complete imaging of the cranial base, maxilla, mandible, the first 4 cervical vertebrae (C1 - C4), and the associated airway. The exclusion criteria were (1) previous orthodontic treatment and/or orthognathic surgery, (2) A point-nasion-B point (ANB) angle < 4°, (3) known syndromic conditions, (4) presence of pathology detectable along the upper airway, and (5) swallowing during scan acquisition.
After application of the inclusion and exclusion criteria, 67 patients were included in the final sample (Fig. 1).
2. Methods
1) CBCT image acquisition
All images were taken by the same operator using the same CBCT device (Alphard-3030; ASAHI Roentgen IND, Kyoto, Japan). The following specifications were used: tube voltage, 80 kVp; dose, 5.0 mA; scanning time, 17 seconds; voxel size, 0.39 mm as cranial mode. All patients were instructed to be seated upright and simultaneously fixed with the chin cup and ear rod to allow Frankfort horizontal to be positioned parallel to the floor. After the images were acquired, they were imported as digital imaging and communications in medicine (DICOM) files using the INFINITT PACS software program (INFINITT healthcare Co., Ltd, Seoul, Korea).
2) Image preparation and airway assessment
3D images were reconstructed from the DICOM files using the OnDemand3D Application (Cybermed, Daejeon, Korea). All CBCT images were reoriented in all three planes according to the following guidelines[9]:
(1)Coronal plane : orbitale of both sides were on the same horizontal plane.
(2)Sagittal plane : Frankfort plane was horizontal.
(3)Axial plane : a line through the crista galli and the basion was vertical.
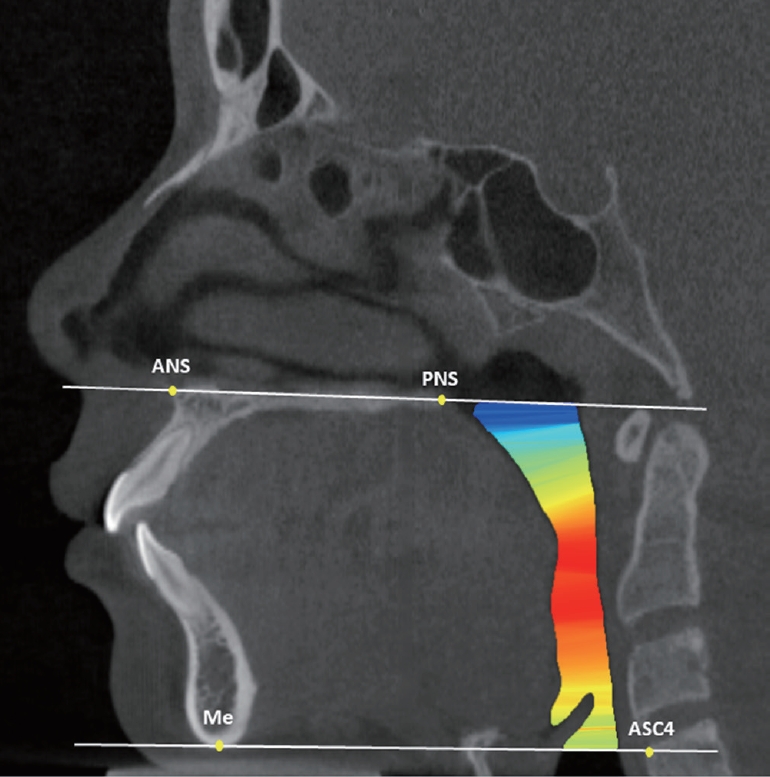
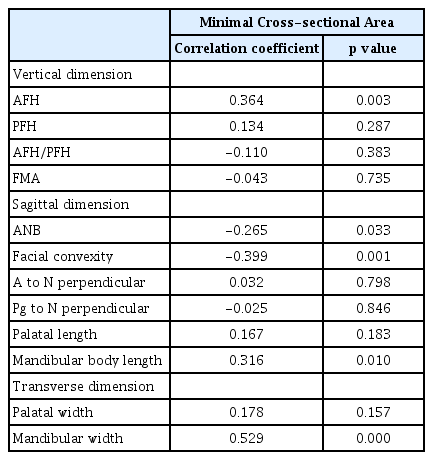
The upper airway volume and minimal cross-sectional area were measured according to the guidelines set forth by Anandarajah et al .[9] (Table 1, Fig. 2). The minimal cross-sectional area reflected the most constricted airway area within the defined margins.
3) Craniofacial morphology assessment
Craniofacial morphology was assessed on automatically constructed 2D lateral and posteroanterior cephalograms with no magnification. The images were imported into VcephTM 6.0 (Osstem Implant, Seoul, Korea) for analysis. The following landmarks and measurements were used in this study:
(1) Landmarks (Fig. 3 and 4)
①Sella (S): The midpoint of sella turcica
②Nasion (N): The most anterior point on frontonasal suture
③Orbitale (Or): The most inferior point on margin of orbit
④Porion (Po): The most superior point of outline of external auditory meatus
⑤Anterior nasal spine (ANS): The apex of the anterior nasal spine
⑥Posterior nasal spine (PNS): The tip of the posterior nasal spine
⑦A-point (A): The most posterior point on the anterior contour of the maxillary alveolar arch
⑧B-point (B): The most posterior point on the anterior contour of the mandibular alveolar arch
⑨Pogonion (Pg): The most anterior point on the mid-sagittal mandibular symphysis
⑩Gonion (Go, lateral cephalogram): The most posterior inferior point on angle of mandible
⑪Gonion (Go, posteroanterior cephalogram): The most lateral point on the convex margin on the angle of mandible
⑫Maxillary notch (Mx): The intersection of the lateral contour of the maxillary alveolar process and the lower contour of the maxillo-zygomatic process of the maxilla
(2) Vertical craniofacial dimensions (Fig. 5)
①Anterior facial height (AFH): The distance between N and Me
②Posterior facial height (PFH): The distance between S and Go
③AFH/PFH: The ratio of AFH to PFH
④Frankfurt-mandibular plane angle: The angle formed by the Frankfurt horizontal plane (Or-Po) and the mandibular plane (Go-Me)
(3) Sagittal craniofacial dimensions (Fig. 6)

Sagittal craniofacial dimensions.
1 = Facial convexity, 2 = A to N-perpendicular, 3 = Pg to N-perpendicular, 4 = Palatal length, 5 = Mandibular body length
①ANB: The difference between sella-nasion-A point and sella-nasion-B point
②Facial convexity: The angle formed by N, A, and Pg
③A to N-perpendicular: The liner distance from A to N perpendicular
④Pg to N-perpendicular: The liner distance from Pg to N perpendicular
④Mandibular body length: The distance between Go and Me
⑤Palatal length: The distance between ANS and PNS
4) Skeletal maturity assessment
Skeletal maturity was assessed on 2D lateral cephalograms using the Cervical Vertebral Maturation index according to Baccetti et al .[10] and categorized as corresponding to prepeak, peak, and post-peak stages.
5) Reliability
This study was conducted by one investigator, and after 4 weeks, 20 patients were randomly selected and remeasured. When the intraclass correlation coefficient (ICC) value was obtained for the measured values of upper airway dimensions and craniofacial morphology, all of them were found to be 0.9 or higher.
6) Statistical analysis
The normality of the distribution was assessed using the Kolmogorov-Smirnov test. Differences in the upper airway dimensions according to gender were analyzed using the MannWhitney test. Differences according to skeletal maturity were analyzed using one-way ANOVA followed by Bonferroni’s Post Hoc test. Pearson’s correlation test was performed to investigate the correlations between upper airway dimensions, age and craniofacial morphology. Partial correlation analysis was performed to eliminate the effects of age and skeletal maturity, and multiple regression analysis was performed to assess the most relevant variables for airway dimensions. All statistical analyses were conducted using Windows SPSS 25.0 (IBM, Armonk, NY, USA).
Ⅲ. Results
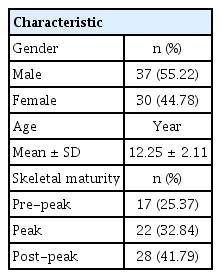
Out of 67 patients, 37 (55.22%) were male and 30 (44.78%) were female. The mean age of the study population was 12.25 ± 2.11 years old. Based on skeletal maturity, 25.7% were in the pre-peak stage, 32.84% were in the peak stage, and 41.79% were in the post-peak stage (Table 2). The mean values for the craniofacial morphology and upper airway dimensions are presented in Table 3.
Gender was not significantly associated with airway volume or minimal cross-sectional area (Table 4). There was a statistically significant difference in the upper airway dimensions according to skeletal maturity. Airway volume increased from pre-peak stage to post-peak stage and showed statistically significant differences between the groups (Fig. 8). The minimal cross-sectional area also increased from pre-peak stage to post-peak stage. No statistically significant differences between pre-peak and peak stages were identified (Fig. 9). Airway volume and minimal cross-sectional area were positively correlated with age (p = 0.000).

Minimal cross-sectional area in relation to skeletal maturity. Bonferroni’s Post Hoc test (* : p < 0.05, NS = not significant)
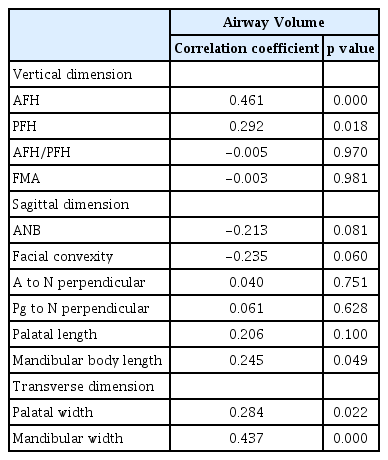
The partial correlation analysis adjusting for age and skeletal maturity revealed significant associations between upper airway dimensions and vertical, sagittal, and transverse craniofacial morphology. Airway volume was positively associated with AFH, PFH, mandibular body length, maxillary and mandibular width (Table 5). The minimal cross-sectional area was positively associated with AFH, mandibular body length, and mandibular width and negatively associated with ANB and facial convexity (Table 6).
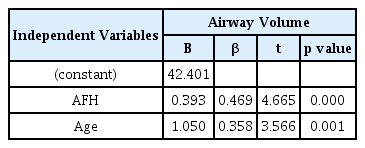
Tables 7 and 8 show the results of multiple regression analysis on upper airway dimensions and variables with confirmed correlation. Upper airway volume showed the highest associations with AFH and age (r2 = 0.502). The variables that showed the highest associations with the minimum cross-sectional area were the mandibular width and age (r2 = 0.544).
Ⅳ. Discussion
Growth and function of the upper airway space are closely associated with maxillofacial growth[11]. The upper airway dimensions have been reported to be influenced by posture, gender, age, obesity, and body mass index[12-14]. Growth-related and anatomical factors can be easily assessed using general orthodontic diagnostic data. It is clinically relevant to investigate which of these factors are most highly associated with upper airway dimension.
The importance and reliability of ANB angle are still controversial[15]. However, it is a commonly used cephalometric parameter in clinical orthodontics[16]. The ANB angle and the angle of convexity in the pre-pubertal assessment showed a high prediction accuracy for post-pubertal jaw relationships[17]; in this study, the ANB angle was used as the scale reflective of the sagittal relationship of the maxilla and mandible.
Anatomical structures vary with growth and development. There exist differences in upper airway assessment between adults and children[18]. In studies where 3D analyses of upper airways were performed in children, there was either a lack of airway delineation according to anatomical boundaries in children[19,20], and/or easily mobile soft-tissue landmarks were used[5,21]. Anandarajah et al .[9] proposed new reliable and reproducible upper airway margins to be used on CBCT scans of children for the assessment of upper airway dimensions.
Most of the studies on upper airway dimensions reported no gender differences[3,4,6,22]. There was no statistically significant gender difference in the airway dimensions in this study. In contrast, Alves et al .[23] reported gender differences in the retropalatal and retrolingual regions in patients with Class III malocclusion. Chiang et al .[24] reported that boys not only had a longer and larger airway than girls but also experienced a faster increase in dimensions.
In this study, upper airway dimensions increased from 8 to 15 years of age and showed a positive correlation with age. This finding is in agreement with the report by Schendel et al .[25], who reported that airway dimensions consistently increased until about 20 years of age. Chiang et al .[24] found that the upper airway dimensions increased during a rapid period of craniofacial growth in patients between the ages of 8 and 18 years.
Although the walls of the upper airway are constructed of soft tissue structures that influence the luminal size, the craniofacial osseous structures determine the general size of the upper airways[18]. Skeletal maturity is closely associated with upper airway dimensions in children. It was found that upper airway dimensions were smallest in patients before pubertal growth, and there was statistically significant differences between growth stages[22,26]. In this study, the upper airway dimensions also increased during growth. These dimensional airway changes in relation to skeletal maturity could reflect the growth-related changes of bony structures surrounding the upper airways. There may be differences in skeletal maturity among the pre-peak, peak, and post-peak groups; future studies will be needed to address such issues.
Many studies have reported the association between sagittal craniofacial dimensions and the upper airway space, with the upper airway space showing a negative correlation with the sagittal intermaxillary relationship[3-6,22]. Just as in previous studies, this study found that upper airway volume and the minimum cross-sectional area were negatively correlated with ANB and facial convexity. However, only the minimum cross-sectional area showed a statistically significant correlation. This may be because this study was conducted in skeletal Class Ⅱ patients, which makes the range of ANB and facial convexity were not significantly dissimilar. Mandibular body length showed a statistically significant correlation with upper airway volume, which was also confirmed in previous studies[3,27].
In relation to vertical craniofacial dimensions, correlations have been found between upper airway dimensions and anterior facial height. This is consistent with the findings of previous studies[3,27]. This indicates that patients with vertical growth patterns with a large anterior facial height are likely to have an expanded airway. However, there were several studies that reported diverse relationships between upper airway dimensions and vertical growth patterns[28,29]. This difference may be due to the fact that there was variability within the study population, and the variables used to assess vertical craniofacial morphology patterns were different from those used in previous studies.
This study identified the association between transverse craniofacial morphology and upper airway dimensions. Maxillary width was found to be positively correlated with upper airway volume. Mandibular width was found to be positively correlated with upper airway volume and the minimum cross-sectional area. Anandarajah et al .[22] reported that mandibular width was significantly correlated with upper airway volume and the minimum cross-sectional area, and that growing patients with a large sagittal intermaxillary relationship and narrow mandibular width are particularly at risk of having narrow upper airway dimensions. In contrast, Di Carlo et al .[30] found no association between upper airway dimensions and craniofacial morphology in all three planes. This discrepancy may be explained by the fact that the patients were scanned in the supine position and the population sample of this study were older than those in other studies.
This study conducted a multiple regression analysis using factors associated with upper airway dimensions. The airway volume was found to have the strongest association with AFH and age. The minimum cross-sectional area was found to have the strongest association with mandibular width and age.
The limitations of this study were that sample size was small and other functional factors influencing the upper airway space were not considered. More comprehensive investigations and large-scale studies should be carried out in the future to overcome the limitations of the present study. Nonetheless, this study conducted a multifactorial analysis of the upper airway dimensions and verified that upper airway dimensions in skeletal Class Ⅱ children and adolescents were associated with age, skeletal maturity, and craniofacial morphology in all three planes.
Ⅴ. Conclusion
This study was conducted to investigate factors influencing the upper airway dimensions in skeletal Class Ⅱ children and adolescents using CBCT images. Upper airway space did not show gender differences, but showed significant associations with age, skeletal maturity, and craniofacial morphology in all three planes. In skeletal Class Ⅱ children and adolescents, the risk of a reduced upper airway space is higher in those who are younger and have smaller anterior facial height and mandibular width.