Ⅰ. Introduction
Resin-modified glass ionomer cement (RMGIC) is a dental restorative material composed of conventional glass ionomer cement (GIC), a photo-activated methacrylate and 2-HEMA or Bis-GMA[
1]. RMGIC has a lower bonding strength than composite resin. RMGIC is one of the restorative materials of choice for dental caries in pediatric patients in the precooperative stage. RMGIC exhibits an excellent microbiological environment at subgingival margin compared to composite resin[
2].
For successful adhesion of restorative materials, hemostatic agents are used to prevent blood contamination. Aluminum chloride (AlCl₃) is one of the most frequently used for bleeding control in the oral cavity[
3,
4]. The occurrence of systemic side effects with aluminum chloride hemostatic agent is less common[
5]. Aluminum chloride hemostatic agent is often used in dental clinics at a concentration of are in 5 - 25% a pH of 0.7 - 3.0[
4,
6,
7]. Aluminum chloride forms a barrier by reacting with blood proteins and arrests the bleeding from the vessels[
8,
9].
Several studies have reported that aluminum chloride hemostatic agents have an additional effect on dentin by removing the smear layer present on the dentinal walls following instrumentation and by changing the composition of the dentinal surface[
6,
10]. Previous studies conducted to investigate the bonding strength of resin restorative material contaminated by aluminum chloride hemostatic agent showed different results according to the use of acid etching and classification of adhesives. One of the studies reported that identified a significantly decreased bonding strength of resin in a group wherein the self-etching adhesive had acted as a hemostatic agent[
11]. Another study confirmed the recovery of bonding strength after using self-etching adhesives followed by acid etching treatment with phosphoric acid[
12]. The document regarding the effect on shear bond strength of dentin of primary teeth by aluminum chloride hemostatic agent is limited.
The purpose of this study was to analyze the effect of aluminum chloride hemostatic agent on the shear bond strength by of RMGIC to dentin of primary molar. Scanning electron microscopy (SEM) based image analysis and energy-dispersive X-ray spectroscopy (EDS) analysis were performed.
Ⅳ. Discussion
The purpose of this study was to investigate the effect of contamination from aluminum chloride hemostatic agent on the shear bond strength of RMGIC to primary tooth dentin.
Many carious lesions are located near the subgingival margin. Contamination with blood and gingival crevicular fluid takes place near the subgingival margin. To prevent such deterioration, practitioners use the hemostatic agent during the restoration process to reduce the amount of gingival crevicular fluid and to control gingival bleeding[
4]. Pediatric dentistry clinics use various hemostatic agents, including aluminum chloride, ferric sulfate, and epinephrine. Each hemostatic agents has a difference in mechanisms for hemostasis. Therefore, the appropriate hemostatic agent is selected for the location and situation of bleeding. The present experiment was performed using an aluminum chloride that acts by precipitating proteins on the superficial layer of mucosa and make it mechanically stronger.
RMGIC is a restorative material composed of GIC with resin components[
1]. The mechanical properties such as tensile strength and compressive strength of RMGIC is equal to those of bond strength GIC. The bonding intensity of RMGIC depends on the ionic bond between hydroxyapatite (HA) of the tooth and restorative material[
13]. Ionic bonding between tooth and material takes place by the acid-base reaction of calcium ions from HA present on the surface of the tooth and carboxyl ion from polyacids present in RMGIC components.
There are previous studies on the negative effects of hemostatic agents on the adhesion of composite resin. It has been reported that ferric sulfate hemostatic agents reduce the bonding strength of composite resin[
12,
14-
17]. Prabhakar and Bedi[
18] suggested that the reduction in shear bond strength may be associated with coagulation of plasma proteins in the dentinal fluid. Another previous studies report that aluminum chloride hemostatic agents have a negative effect on bonding strength of composite resin[
10,
12,
14,
15]. It was postulated that insoluble complex was responsible for the decrease in bonding strength on the composite resin which contaminated with aluminum chloride[
19]. In the case of two hemostatic agents, some researchers report that the reduced bonding strength was recovered when using the total etching adhesive system[
10-
12,
20]. In primary tooth, it also showed a tendency to decrease bonding strength due to hemostatic agents[
16,
18]. Our findings, using RMGIC, were in line with those of the previous studies which report the decrease in bonding strength after the application of hemostatic agents.
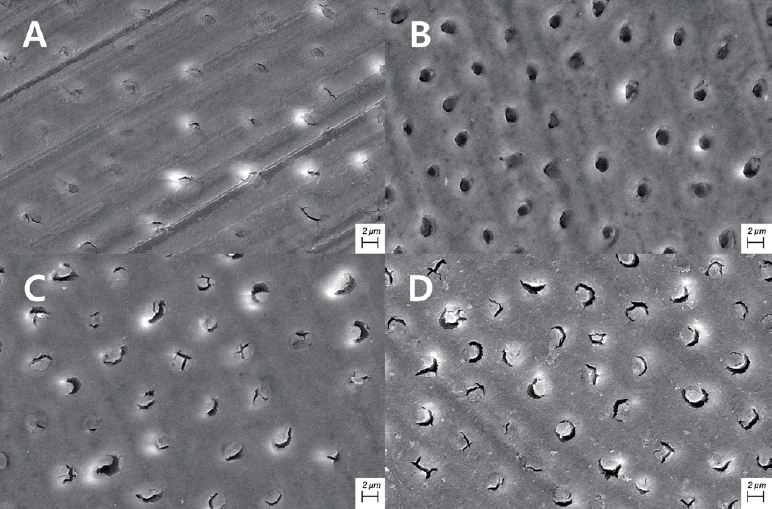
The micromechanical bonding of polymers that penetrate the dentinal tubules is important[
21,
22]. The polymer penetrating the dentinal tubules exposed after elimination of the smear layer enhances the micromechanical bonding strength by increasing the surface area. Elimination of the smear layer observed in the SEM after the use of aluminum chloride hemostatic agent was consistent with the previous study[
6,
23,
24]. Land
et al.[
6] reported that the smear layers partially eliminated within 30 seconds to 5 minutes after the application of aluminum chloride hemostatic agent. The amount of smear layer elimination was increased by time. However, the openings of dentinal tubules were not fully exposed, and debris remained within the dentinal tubules.
In the present study, the shear bond strength of group III was significantly lower than that of group I. Hemostatic agent use may have contributed to the reduction in the bond strength between dentin and RMGIC. If the aluminum ion contained in the aluminum chloride hemostatic agent reacts with HA of the tooth surface, it is replaced by calcium ions of HA to form an insoluble complex, Al(OH)₂H₂PO₄[
19]. Accordingly, it reduces the reactivity of the dentin surface and leads to decreased ionic bond with RMGIC.
According to Saad
et al.[
14], there was no significant difference in bond strength between a group involving RMGIC restoration wherein PAA was applied after contamination with an aluminum chloride hemostatic agent and a group involving RMGIC restoration wherein PAA was applied without the use of a hemostatic agent. However, it was significantly lower than the group that only applied PAA without hemostatic agent. Such difference in results seems to be due to the differences in constituents of the material and type of the teeth used in the study.
As a result of EDS analysis, aluminum was identified in groups III and IV which was not present in groups I and II. Groups III and IV showed the similar weight percentage of aluminum. Weight percentage of chlorine in group III was higher than in groups I, II and III. This indicates that the application of PAA has reduced the weight percentage of chlorine on the tooth surface, whereas the effect was minimal in the case of aluminum. This result is consistent with those of the previous studies. This was associated with reduced calcium after using a hemostatic agent and is assumed to be the result of complex formation.
Application of 37% phosphoric acid following the use of aluminum chloride hemostatic agent can effectively eliminate some of the remaining smear layers due to acid corrosion. And the openings of the dentinal tubules were observed more clearly following its application[
11,
24]. In addition, one study using ethylene diamine tetraacetic acid reports that the bonding strength was restored[
10]. But, many studies that used PAA following the use of aluminum chloride hemostatic agent reported that this protocol was inadequate to effectively remove the debris remaining within the dentinal tubules[
14,
23]. The findings from the previous studies are consistent with the SEM results from the present study.
In this study, the experiment was conducted to verify alterations in tooth surface and reduction in the bond strength by a hemostatic agent. One previous study on this reduction suggests cutting off the portion of surface dentin that was exposed to the hemostatic agent[
25]. Additional studies need to be conducted to explore ways to recover the bond strength of dentin contaminated with aluminum chloride as much as that of the uncontaminated surface during the process of bonding RMGIC.
The present study examined the influence of aluminum chloride. In addition to aluminum chlorides, a variety of hemostatic agents such as ferric sulfate are used in pediatric dental clinics. Further studies are required to compare and analyze the effects of those various hemostatic agents on bonding strength between RMGIC and dentin of primary teeth.
Since there are restoration cases using RMGIC for permanent tooth in the department of pediatric dentistry such as amelogenesis imperfecta cases and erupting tooth, a study investigating the effect of aluminum chloride hemostatic agent on permanent dentin is also required.













 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print