Silver Diamine Fluoride와 요오드화 칼륨 도포 후 상아질 표면 거칠기와 초기 우식원성 세균막 형성
Surface Roughness of Dentin and Formation of Early Cariogenic Biofilm after Silver Diamine Fluoride and Potassium Iodide Application
Article information
Abstract
이 연구는 우식원성 세균막 형성과 상아질 표면 거칠기에 대한 Silver diamine fluoride와 요오드화 칼륨(KI)의 영향을 실험실 환경에서 평가하는 것이다.
인공 우식을 유발한 48개의 Bovine dentin 시편을 제작하여 대조군, SDF 도포, SDF와 KI 도포(SDFKI)의 3개 군으로 나누었다. 각 군 당 10개의 시편의 상면에 Streptococcus mutans, Lactobacillus casei 및 Candida albicans 를 포함하는 다종 우식원성 세균을 24시간 동안 배양 후 세균 수를 측정하였다. 나머지 시편은 원자힘 현미경과 주사전자현미경으로 관찰하였다.
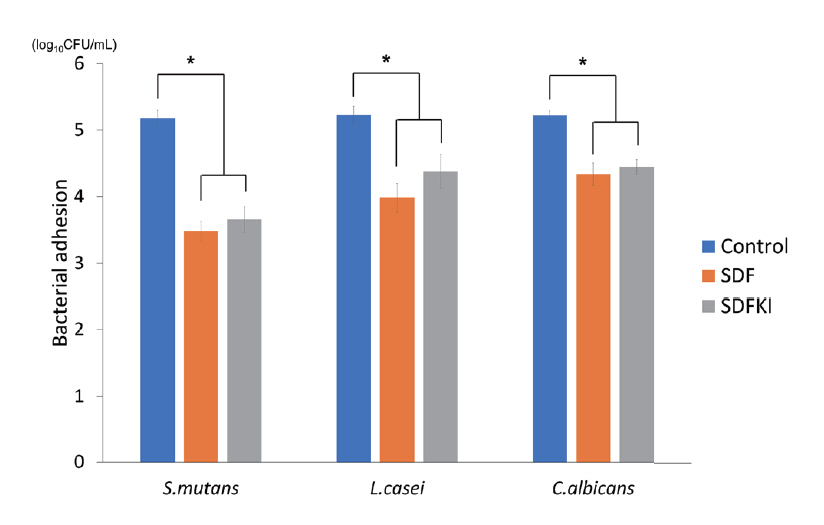
SDF와 SDFKI 도포 시 대조군에 비해 세균 수가 유의하게 감소했으며, KI는 SDF의 항균 효과를 유의하게 저해하지 않았다. 현미경 관찰 시 SDF와 SDFKI 도포 후 생성되는 입자가 상아질 표면에 침착 되는 것을 관찰했으나 표면 거칠기는 세 군 간 유의한 차이가 없었다.
Trans Abstract
This study aimed to evaluate the effect of silver diamine fluoride (SDF) and potassium iodide (KI) on the formation of cariogenic biofilm and surface roughness in vitro.
A total of 48 bovine dentin specimens with artificially induced caries were prepared and divided into 3 groups of 16: untreated control, SDF-treated, and SDF-treated followed by KI (SDFKI). Ten specimens from each group were used to observe microbial adhesion. Multispecies cariogenic biofilms including Streptococcus mutans, Lactobacillus casei, and Candida albicans were cultured on the specimens. Microbes were cultured for 24 hours, and the colony-forming unit was calculated. The remaining specimens were observed by atomic force microscope and scanning electron microscope (SEM).
The number of bacteria was significantly lower in the SDF and SDFKI groups. KI did not inhibit the antibacterial activity of SDF significantly. SEM images showed particles generated after SDF and SDFKI application were deposited on the dentin, but there was no significant difference in surface roughness between the 3 groups.
This study confirmed that SDF and SDFKI application did not have a significant effect on the surface roughness of dentin, but effectively inhibited the formation of the early cariogenic bacterial film after 24 hours compared to the control.
Ⅰ. Introduction
Dental caries is the most common chronic disease in children, and its standard treatment is to remove the demineralized tissue and fill it with restorative materials[1]. However, many patients find it difficult to receive proper dental treatment at the right time due to fear, anxiety, access to care and financial problems[2]. The study of the global burden of oral condition[3] conducted in 2010 indicated that untreated dental caries in children was the 10th most common among 291 oral diseases evaluated. The application of silver diamine fluoride (SDF) has been proposed as an alternative approach to the treatment of dental caries in children.
SDF is a colorless and basic liquid that contains approximately 24 - 28% silver and 5 - 6% fluoride[4,5]. Silver has an antimicrobial effect and fluoride promotes remineralization[6]. The application of SDF is a simple and economical treatment, which corresponds to the concept of minimally invasive dentistry[7]. However, the black staining that occurs after SDF application is a barrier to its clinical application.
To solve the discoloration caused by SDF, a method of applying potassium iodide (KI) immediately after SDF application has been proposed recently. KI prevents staining by depositing excess silver ions as white silver iodide[8]. Nguyen et al.[9] reported that the application of SDF followed by KI (SDFKI) resulted in little to no darkening, compared to applying SDF only.
To prevent new caries from occurring on the tooth surface coated with SDF and SDFKI, the formation of a cariogenic biofilm should be effectively managed by the applied agent[10]. The antibacterial activity of the drug itself and the change in surface roughness after the application of the drug may affect the formation of the biofilm. Bollen et al.[11] reported that when the surface roughness is greater than 0.2 μm, more bacteria can attach to the surface. Although it was reported that Streptococcus mutans biofilm was inhibited after SDF application[12,13], studies on the effect of SDFKI application on the formation of the bacterial film are rare, and there have been no studies using multispecies bacteria. In addition, there are few studies on the surface roughness of dentin coated with SDFKI.
Therefore, this study aimed to evaluate the effect of SDF and SDFKI applied to demineralized dentin on the formation of multispecies early cariogenic biofilms and surface roughness of dentin under laboratory conditions.
Ⅱ. Materials and Methods
1. Preparation of dentin specimen
The enamel of the bovine incisor was removed. The dentin specimen was prepared by using a low-speed diamond disk to cut the bovine incisor under water to a size of 3.0 × 3.0 × 2.0 mm and polishing the surface with 1000 grit sandpaper. To observe the upper surface of the dentin specimen, the side and rear surfaces of the dentin specimens were blocked using silicone (Examixfine injection type, GC Corporation, Tokyo, Japan, Fig. 1). A total of 48 specimens were prepared and sterilized using ethylene oxide gas.
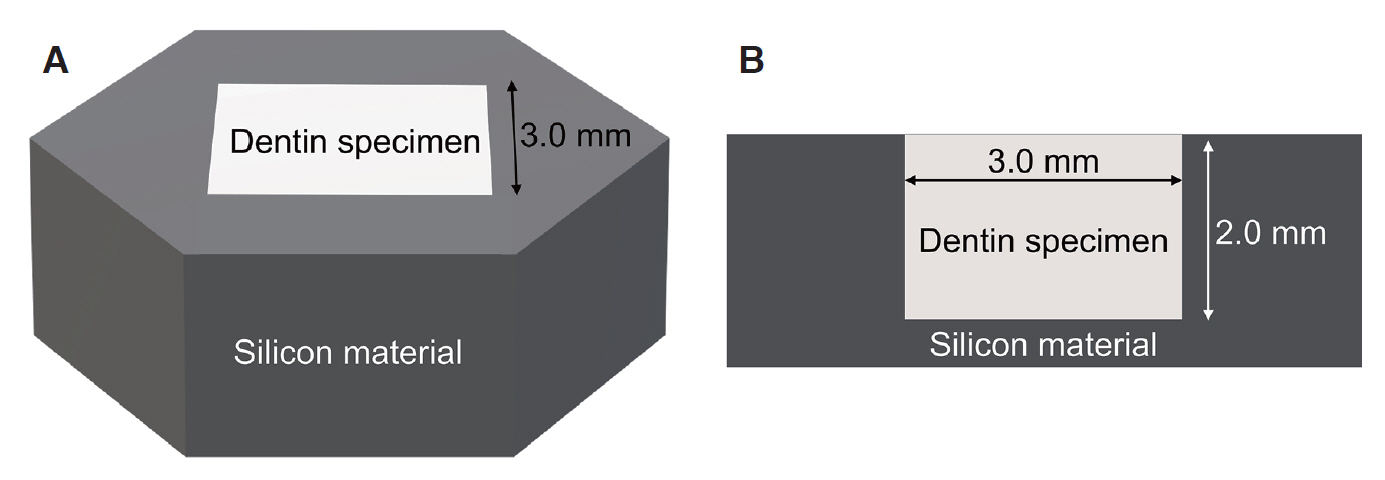
Preparation of dentin specimens. (A) Stereoscopic view of the specimen, (B) Cross sectional view of the specimen.
To induce artificial caries on the upper surface of the dentin specimen, an acetic acid demineralization solution was prepared, as described in a previous study[14]. A demineralization solution was prepared by mixing 50 mM acetic acid, 3 mM CaCl2 × H2O, 3 mM KH2PO2, and 6 mM methylhydroxydiphosphonate and followed by titrating to pH 4.95 using 10% HCl and 10 M KOH. Subsequently, the solution was sterilized by filtering (0.22 μm vacuum filter system, JetBiofil®, Guangzhou, China). The dentin specimens were placed in a sterilized demineralization solution and stored in a water bath at 37°C for 7 days.
2. Treatment of dentin specimens
Riva Star™ (SDI, Bayswater, Australia), the SDF and KI formulation recently approved by the Korean Ministry of Food and Drug Safety, was applied to the dentin specimen according to the manufacturer’s instructions. Riva Star™ consists of 2 agents. The 1st is 30 - 35% SDF, and the 2nd is a saturated solution of KI[15]. It was developed to prevent black discoloration by applying a sufficient amount of KI immediately after the application of SDF.
The 48 specimens prepared were divided into 3 groups of 16: untreated control, SDF-treated (SDF), and SDF-treated followed by KI (SDFKI). In the SDF group, 1.0 μL of Riva Star™ agent 1 (RSA-1) was evenly applied to 16 specimens for 10 sec with a sterile superfine-sized microbrush. In the SDFKI group, 1.0 μL of RSA-1 was applied, and 1.0 μL of Riva Star™ agent 2 (RSA-2) was immediately applied to 16 specimens with a sterile superfine-size microbrush for 10 sec. RSA-2 was applied twice to the specimens to ensure that KI was sufficiently applied. All specimens in the SDF and SDFKI group were washed with sterile distilled water 3 minutes after the treatment.
3. Bacterial culture
S. mutans ATCC 25175, Lactobacillus casei ATCC 334, and Candida albicans ATCC 18804 standard strains were inoculated in Brain Heart Infusion broth (BHI broth; Becton, Dickinson and Company, Sparks, MD, USA) and incubated under aerobic conditions, supplemented with 5% CO2 at 37°C for 18 hours. The turbidity of each bacterial culture was measured using a spectrophotometer (X-ma 1200, Human Corporation, Seoul, Korea) and then diluted to 2 × 109 colony-forming unit (CFU)/mL using BHI broth for inoculation.
4. Formation of cariogenic biofilm
Thirty bovine dentin specimens, 10 from each group, were used to observe microbial adhesion. The specimens were placed in a well plate with 1390 μL of BHI broth containing 1% sucrose, as well as 300 μL of S. mutans, 300 μL of L. casei, and 10 μL of C. albicans. The final inoculation concentrations of each bacterium were 3 × 108 CFU/mL of for S. mutans and L. casei and 1 × 107 CFU/mL for C. albicans. This concentration was set based on a pilot study to obtain a similar colony number for the measurement of the final bacterial count. The specimens were cultured for 24 hours at 5% CO2 and 37°C.
5. CFU measurement
Specimens cultured for 24 hours were washed twice with phosphate-buffered saline (PBS) and then sonicated for 10 s (VC 100, Sonics & Materials Inc., Danbury, CT, USA) to obtain a bacterial suspension. A bacterial suspension of 50.0 μL diluted with PBS was smeared on a blood agar plate (Hanil-KOMED, Seongnam, Gyeonggi-do, Korea) and cultured for 72 hours at 5% CO2 and 37°C. The number of bacterial CFUs was counted by the naked eye. Each species was distinguished by the morphology of the bacterial colony. The number of bacterial CFUs was converted into logarithms and analyzed.
6. Microscopic observation
The remaining 6 specimens from each group were observed by microscope. Three specimens from each group were observed using an atomic force microscope (AFM, PSIA XE-100, Park Systems, Suwon, Korea). Surface roughness was measured at 3 random points in the center of each specimen, and the roughness (Ra value) was calculated by averaging the measured values. For the qualitative evaluation of the surface of the specimen, the remaining specimens were observed using a scanning electron microscope (SEM, JSM-IT500, JEOL, Tokyo, Japan).
7. Statistical analysis
Statistical analysis of log CFU/mL values was performed using IBM SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). The Kruskal-Wallis test was performed on the measured CFU and Ra values for each group to verify statistical significance, followed by Bonferroni post-hoc analysis.
Ⅲ. Result
1. Bacterial adhesion
Compared with the control group, the number of bacterial CFUs was significantly lower in the SDF and SDFKI groups. For SDFKI group, the CFU value was larger than that in SDF group, but this was not statistically significant (Table 1, Fig. 2).
2. Surface roughness
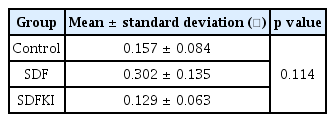
Changes in the surface roughness caused by the application of SDF and SDFKI were observed by AFM (Fig. 3). The Ra value of the SDF group was the largest, but there was no statistically significant difference between the 3 groups (Table 2).
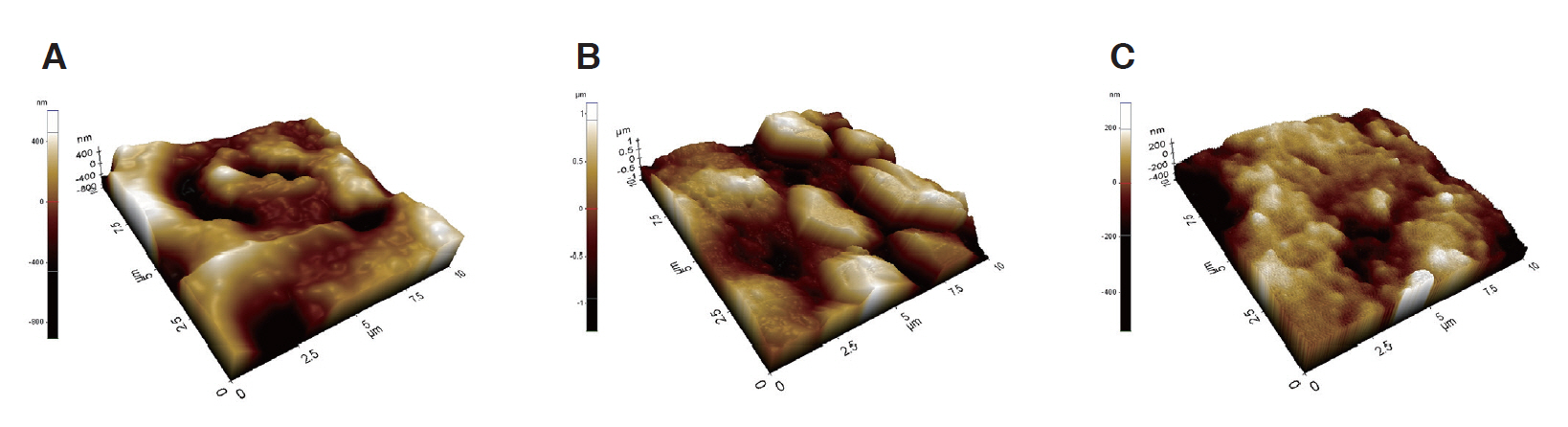
Atomic force microscope imaging of surfaces. (A) Control, (B) SDF application, (C) SDFKI application.
SDF = Silver diamine fluoride, SDFKI = Silver diamine fluoride and potassium iodide
3. Quality of dentin surface
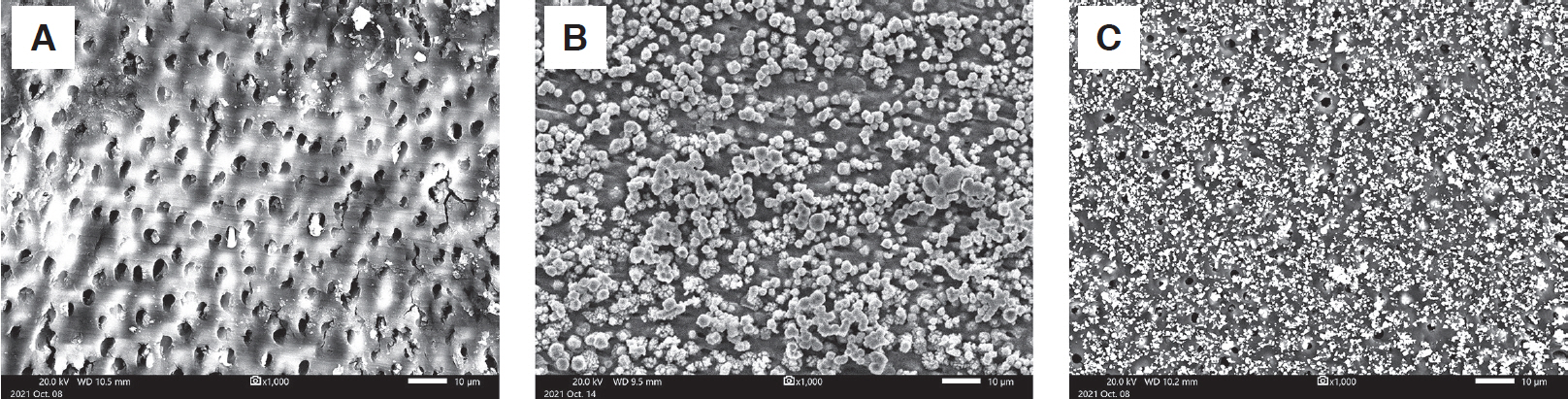
SEM confirmed that the particles produced when applying SDF and SDFKI accumulated on the demineralized dentin surface and dentinal tubules. The particles observed on the dentin surface coated with SDFKI were smaller than those on the SDF coated surface (Fig. 4).
Ⅳ. Discussion
SDF contains silver and fluoride that can inhibit the formation of cariogenic bacterial films. It was known that 38% of SDF contains 44800 ppm fluoride[5]. This concentration is the highest of any currently available fluoride product. High concentrations of fluoride can bind to bacterial cell components and affect enzymes involved in carbohydrate metabolism and sugar absorption, thereby inhibiting the formation of a bacterial film[16]. Silver ions can inhibit electron transport systems of bacteria, damage cell membranes, as well as cause DNA mutations and cell death[17].
The black staining visible after the application of SDF is due to the precipitation of silver phosphate[16,18]. When immediately after SDF, KI reacts with the free silver ions in SDF and prevents the formation of silver phosphate[19]. Detsombonnrat et al.[20] observed a significant immediate reduction in staining due to SDF following application of KI in a dose dependent manner[20].
In clinical practice, application of 5.0 μL of SDF per tooth for 1 - 3 min after drying the tooth surface with compressed air is recommended[21]. The amount of SDF applied in this study was 1.0 μL, considering the size of the specimen. In a previous study conducted on specimens of the same size[22], a potent antibacterial effect was obtained by applying 1.0 μL of SDF. The amount of KI used in this study was twice that of SDF. The manufacturer’s manual recommends applying sufficient KI until discoloration disappears after SDF application. In this regard, Riva Star™ formulation was produced in twice the amount of the RSA-2, than the RSA-1. The amount of KI was determined with reference to this, and the black discoloration did not appear in the specimens coated with SDFKI during the experiment.
Dental caries results from interactions between acid-producing bacteria and many host factors over time, including teeth and saliva[1]. A caries lesion develops on a specific surface of the tooth, under the mature dental biofilm coating it for a long period[23]. In this study, 3 types of bacteria highly correlated with the occurrence of the dental caries were used for the formation of multispecies cariogenic biofilms. S. mutans and Lactobacillus species play important roles in the development and progression of caries, respectively[24]. In addition, C. albicans had been reported to be highly correlated with the decay-missing-filled score[25], and early childhood caries[26].
It was confirmed that compared to the control group, the formation of multispecies early cariogenic bacterial film was significantly inhibited when SDF was applied to the dentin specimen in this study. The inhibitory effect of SDF on the biofilm in this study was lower than that in previous studies. Vinson et al.[27] and Mei et al.[4] had reported a 7-fold log10 reduction in bacterial CFU after application of SDF. The concentration of SDF may have contributed to this result. The RSA-1, the formulation used in this study, was 30 - 35% SDF, in which the concentration of fluoride and silver ions was relatively lower than that of the 38% SDF used previously[4,12,13,27]. In addition, the use of demineralized dentin specimens in this study, and the different composition of multispecies cariogenic bacteria may have also had impact on the result.
The formation of an early biofilm was also inhibited after SDFKI application compared to the control group. The inhibition ability of the biofilm formation in the SDFKI group was smaller than that in the SDF group, but there was no statistically significant difference. Takahashi et al.[28] reported that when SDFKI was applied, the formation of S. mutans bacterial films significantly increased because silver particles might be washed more easier compared to the application of 38% SDF. It is also suggested that the application of the KI solution might reduce the number of silver ions[29]. In contrast to this, the use of lower concentration SDF than in previous study[28,29] and multispecies bacteria might contributed to the result of this study. Biofilms comprising multiple bacterial species are more resistant to antibacterial treatment than when comprising a single species because of their thickness, complexity of structure, and interactions between bacteria[30]. Culturing the specimens in the well-plate may have also led to the difference in results. When the specimens were cultured under a flow system using an oral biofilm reactor as in the previous study[28], it may become difficult for bacteria to adhere to the specimen.
Among the 3 cariogenic bacteria, the inhibitory effect on the S. mutans biofilm was the greatest, and the inhibitory effect on C. albicans was the least. Mei et al.[4] also reported that the S. mutans biofilm was most effectively inhibited among the multispecies bacteria when SDF was applied. Silver can inactivate and interfere with the synthesis of glucans and fructans, which are key polysaccharides for the formation and proliferation of S. mutans[27]. C. albicans is a fungus with characteristics different from those of bacteria. This may have influenced the results of this study, but additional studies are needed to determine the exact cause.
Surface analysis by SEM showed that particles were deposited on the dentin specimen after SDF and SDFKI application. In the SDF group, larger particles were observed than in the SDFKI group, covering a greater number of dentinal tubules. Greater surface roughness was observed by AFM in the SDF group, but no statistical significance was found between any of the groups. The large size of the particles generated in the SDF group may have affected the greater surface roughness. Sayed et al.[18] also reported that the surface roughness increased from day 2 in the group treated with SDF. Surface roughness and adhesion of microorganisms usually have a positive correlation[31,32], but in this experiment, a decrease in microorganisms was observed when SDF was applied. This is probably because the particles generated after the application of the drug effectively blocked the penetration of bacteria into dentinal tubules[15], and the antibacterial effect of the drug itself is large.
The scope of this study is limited in that it was conducted in vitro for a short period of time. As mature biofilms have high cell density and complex structures[33], the effects of SDF and SDFKI may differ from those observed. An actual oral environment is a place where more bacteria interact, and various stimuli are transmitted. Further studies in an environment similar to that of the oral cavity are warranted. In addition, as bovine teeth were used in this study, the degree or pattern of demineralization by artificial caries solution may have differed from those of human teeth.
The findings of this study are significant as it employed Riva Star™ which increased the possibility of clinical application of SDF by reducing discoloration. When both RSA-1 and RSA-2 were applied to the dentin, the dentin was maintained without black discoloration during the experiment. Although a greater inhibitory effect on bacterial film formation was observed in the group treated with SDF alone, it was not statistically significant compared to the group treated with SDFKI; thus, it may be used to prevent black pigmentation while obtaining the effect of SDF.
Ⅴ. Conclusion
When SDF and SDFKI were applied to demineralized dentin, there was no significant change in surface roughness, but the formation of the early cariogenic biofilm was suppressed. KI application immediately after SDF did not interfere with the antibacterial effect of SDF significantly.