Premixed MTA제재의 pH, 이온 유리 정도, 용해도
pH, Ion Release Capability, and Solubility Value of Premixed Mineral Trioxide Aggregates
Article information
Abstract
이 연구는 premixed MTA 제재와 기존 치수복조제의 경화 전, 후의 pH 값 그리고 칼슘, 황, 스트론튬 이온의 유리량, 용해도를 비교했다. 사용된 재료는 다음과 같다 : 레진 강화형 칼슘실리케이트(TheraCal LC®; TLC), 레진 강화형 수산화칼슘(UBP, UltraBlendTM plus), 2종류의 premixed MTA(Endocem MTA® premixed regular 〔EMPR〕 and Well-RootTM PT 〔WRP〕). 각 재료의 시편은 경화 전, 경화 후 2군으로 나누어 준비한 뒤 증류수에 보관하였다. pH, 용해도를 측정하였으며 ICP-AES를 이용한 칼슘, 황, 스트론튬의 3가지 이온 유리량을 측정하였다. 경화 후 군에서 TLC와 UBP의 pH 값은 감소했다. 그러나 premixed MTA 재료의 pH 값은 증가했다. TLC는 다른 재료와 비교하여 스트론튬 이온 유리량이 더 많았다. 동시에 EMPR에서 황이온 유리량이 높았다(p < 0.05). 경화 후 군에서 칼슘 이온 방출은 두 종류의 premixed MTA에서 더 높았다(p < 0.05). 경화 후 군에서 용해도는 Kruskal-Wallis 서 test를 이용하여 통계분석하였고 Mann-Whitney U test를 이용하여 사후검정하였다. 결론적으로 레진 강화형 칼슘 실리케이트 시멘트, 레진 강화형 수산화칼슘 시멘트, 2종류의 premixed MTAs 모두 경화 후 알칼리성 pH 값과 낮은 용해도를 가지고 있었으며 다양한 이온을 유리했다.
Trans Abstract
The current study aimed to compare the pH, solubility value, and ion release capability of premixed mineral trioxide aggregates (MTAs) versus conventional pulp capping materials before and after setting. The following materials were used: resin-modified calcium silicate cement (TheraCal LC®, TLC), resin-modified calcium hydroxide cement (Ultra-BlendTM plus, UBP), and 2 kinds of premixed MTA (Endocem MTA® premixed regular 〔EMPR〕 and Well-RootTM PT 〔WRP〕). The specimens of each material were prepared before and after setting and were immersed in distilled water. The materials’ pH and solubility value were assessed. Next, three kinds of ion (calcium, sulfide, and strontium) released by pulp capping materials were evaluated via inductively coupled plasma atomic emission spectrometry. In the after-setting group, the pH of TLC and UBP decreased. However, the pH of the premixed MTAs increased with time. TLC released a higher concentration of strontium ion compared with the other materials. Meanwhile, EMPR released a significantly high concentration of sulfide ion (p < 0.05). In the after-setting group, the 2 kinds of premixed MTAs released a significantly higher concentration of calcium ion compared with the other materials (p < 0.05). In the after-setting group, EMPR had a significantly low solubility value (p < 0.05). The Kruskal-Wallis test, followed by the Mann-Whitney U test with Bonferroni correction, was used in statistical analysis. In conclusion, resin-modified calcium silicate cement, modified calcium hydroxide cement, and the 2 kinds of premixed MTAs had an alkaline pH and low solubility value and they released various concentrations of ions after setting.
Introduction
Root canal treatment of immature teeth with an open apical foramen is associated with a high fracture risk and stops root development. Vital pulp therapy maintains root development and initiates dentinogenesis for pulp protection[1]. In immature teeth with reversible pulpitis caused by caries or trauma, pulp therapy can suppress pulp inflammation and return the pulp to its normal state[2].
Calcium hydroxide, which releases hydroxide and calcium ions, has been the most widely accepted pulp capping material since the 1930s[3-5]. As calcium hydroxide dissolves over time and forms a necrotic layer at the pulp-material interface, failure of long-term biological sealing, which can prevent bacterial infection, commonly occurs[5-7].
Several studies have introduced the use of mineral trioxide aggregates (MTAs). Results showed that the properties of MTAs are superior to those of calcium hydroxide. That is, MTAs have a low cytotoxicity risk and solubility value and good tissue biocompatibility and can promote marginal adaptation and mineralized tissue formation[8-10]. However, these materials have several disadvantages. For example, they have a long setting time and are complicated to manipulate and associated with a risk of discoloration[8,11]. Inhomogeneous mixing may deteriorate the physicochemical properties of MTAs leading to prolonged treatment time[12-15].
To improve the handling characteristics of conventional MTAs have been developed, premixed type MTAs, which are a combination of calcium phosphate cement powders and a nonaqueous but water-miscible liquid. Premixed MTAs have several additives such as methylcellulose or glycerin, which is used as anti-washout ingredients, and calcium chloride or zirconium oxide, which is used as an accelerator. Therefore, they have better material cohesiveness and plasticity, thereby making them easier to handle and accelerating their setting time[16-19]. Ready-to-use cement pastes, such as Well-RootTM PT (WRP, Vericom Co., Chuncheon, Korea) and Endocem MTA® premixed regular (EMPR, Maruchi, Wonju, Korea), have no risk of inhomogeneous mixing[14,15]. WRP comprises glycol and zirconium oxide, and EMPR consists of dimethyl sulfoxide, hydroxypropyl methylcellulose and zirconium oxide, which improve its physicochemical properties. Current studies on premixed MTAs are limited.
In clinical settings, resin-modified materials such as TheraCal LC® (TLC, Bisco Inc., Schaumburg, IL, USA) and Ultra-BlendTM plus (UBP, Ultradent Products Inc., South Jordan, UT) are often applied to deep cavities[20]. Incomplete curing can easily occur and can decrease physical properties and increase toxicity risk[21-25]. However, there are only few studies assessing these issues.
The physicochemical properties of pulp capping materials, such as pH, solubility value, and ion release capability, can affect pulpal response[26,27]. An acidic pH disrupts protein synthesis and proliferation of pulp cells[27,28]. Additionally, calcium ions play an important role in the differentiation of pulp cells into mineralized tissue forming cells[29,30]. Therefore, a high solubility can exacerbate pulp inflammation due to bacterial infection[4,5,31]. The current study aimed to compare the pH, solubility, and ion release capability of WRP and EMPR versus TLC and UBP before and after setting.
Materials and Methods
1. Sample preparation
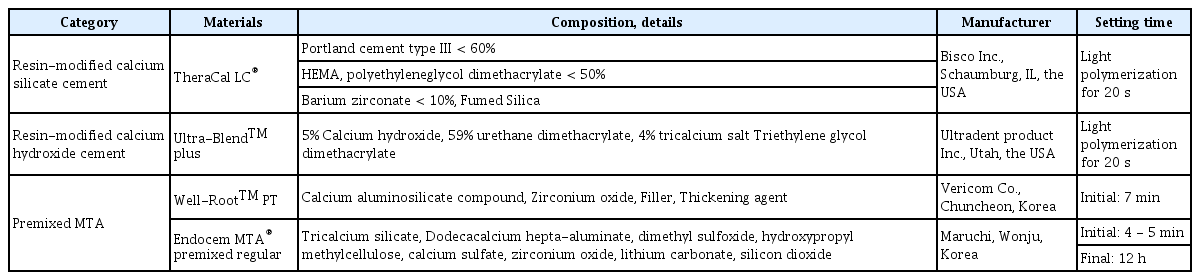
This study used 4 pulp capping materials (Table 1). All materials were evaluated before and after setting in all experimental procedures, and the setting was performed according to the manufacturer’s instructions.
To measure pH and ion release with ICP-AES, 1 specimen of each variable was made for each group, where they were mixed and placed onto circular Teflon molds (inner diameter: 10.0 mm and height: 2.0 mm). To measure solubility value, 2 specimens were made for each group. Each specimen was mixed and placed in split-ring Teflon molds (inner diameter: 20.0 mm, height: 1.0 mm).
2. pH
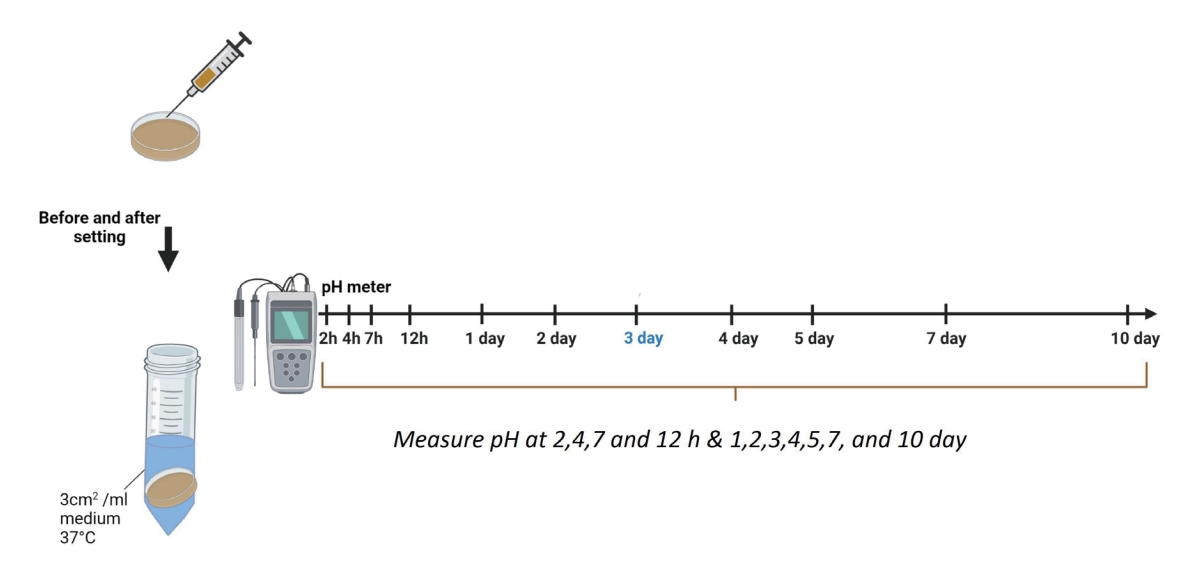
Each sample was placed into a separate tube containing 5.0 mL of distilled water. The samples were stored at 37°C. pH was measured after incubation for 2, 4, 7, and 12 h and for 1, 2, 3, 4, 5, 7, and 10 days using a pH meter (inoLab pH 7110, WTW, Weilheim, Germany), which was previously calibrated with pH 10.0, 7.0, and 4.01 solutions. The electrode was washed with distilled water between each measurement and was blot dried. Each sample was measured 5 times and the mean value was recorded (Fig. 1).
3. Ion release with inductively coupled plasma atomic emission spectrometry
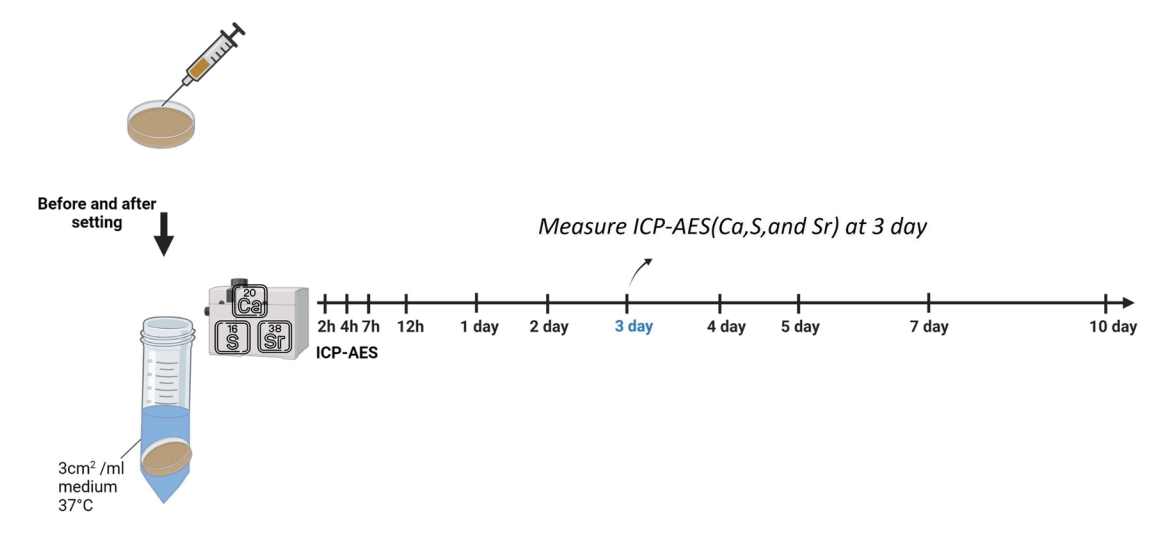
The release of calcium, sulfide, strontium ions was evaluated using the extract with ion release with inductively coupled plasma atomic emission spectrometry (ICP-AES) (Optima 4300DV, Perkin-Elmer, Waltham, MA, USA), according to a previous protocol[32]. Each sample was evaluated thrice, and the mean value was recorded (Fig. 2).
4. Solubility value
The solubility tests were modified based on the standard ISO 6876[33]. The filled molds were stored in an incubator with a temperature of 37℃ and a relative humidity of 100% for 24 hours. The specimens were removed from the molds, and the net weight was evaluated thrice. Then, the average reading was recorded to the nearest 0.001 g with a precision scale. The 2 specimens were immersed in 50 mL of distilled water in the shallow dish for the entire surface of the specimen to allow contact with the distilled water. After 24 hours, the specimens were removed from the dish, and eluted distilled water was poured into a funnel in a beaker. The water was evaporated without boiling and dried in an oven at 110℃. The differences between the specimen’s weight and the beaker’s original weight were divided by the initial weight of the specimen and were multiplied by 100. The result was considered as the solubility.
The solubility test was repeated thrice and conducted again at 1 week using the same method (Fig. 3).
5. Statistical analysis
All data from the repeated tests were presented as mean ± standard deviation. Data were analyzed using the Kruskal-Wallis test and the Mann-Whitney U test with Bonferroni correction using the Statistical Package for Social Sciences software version 21.0 (SPSS Inc., Chicago, USA).
Results
1. pH
Fig. 4 shows the pH of the test materials at different time points. WRP and EMPR had significantly higher pH values than TLC and UBP in all measurement periods (p < 0.05). In the after-setting group, the pH of WRP and EMPR significantly increased over time until after 1 day and then became constant. Moreover, the pH of TLC and UBP significantly decreased over time until 12 hours and became constant (p < 0.05).

Mean pH values after 2, 4, 7 and 12 h and 1, 2, 3, 5, 7, and 10 days of exposition to all tested dental capping materials (n = 5). (A) pH of the before setting group, (B) pH of the after setting group, (C) pH of the before setting group within a day, (D) pH of the after setting group within a day. Asterisks (*) indicate statistically significant differences between the 2 resin-modified materials and 2 premixed MTAs (p < 0.05).
TLC = TheraCal LC®, UBP = Ultra-BlendTM plus, WRP = Well-RootTM PT, EMPR = Endocem MTA® premixed regular, n= number of measurements.
WRP and EMPR had similar patterns before and after setting. However, the pH of TLC and UBP changed irregularly before setting. All materials had an alkaline pH at all periods.
2. Ion release
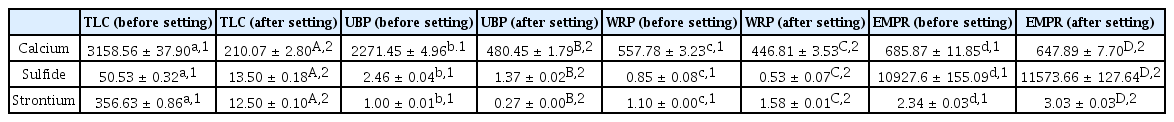
Fig. 5 and Table 2 depict the concentration of ion released assessed via ICP-AES. TLC released a significantly higher concentration of ion before setting than after setting (p < 0.05). Results showed that in the after setting group, WRP and EMPR released a significantly higher concentration of calcium ions compared with TLC and UBP (p < 0.05). EMPR released the highest concentration of sulfide ions before and after setting (p < 0.05). Small concentrations of strontium ions were detected in all materials except TLC.
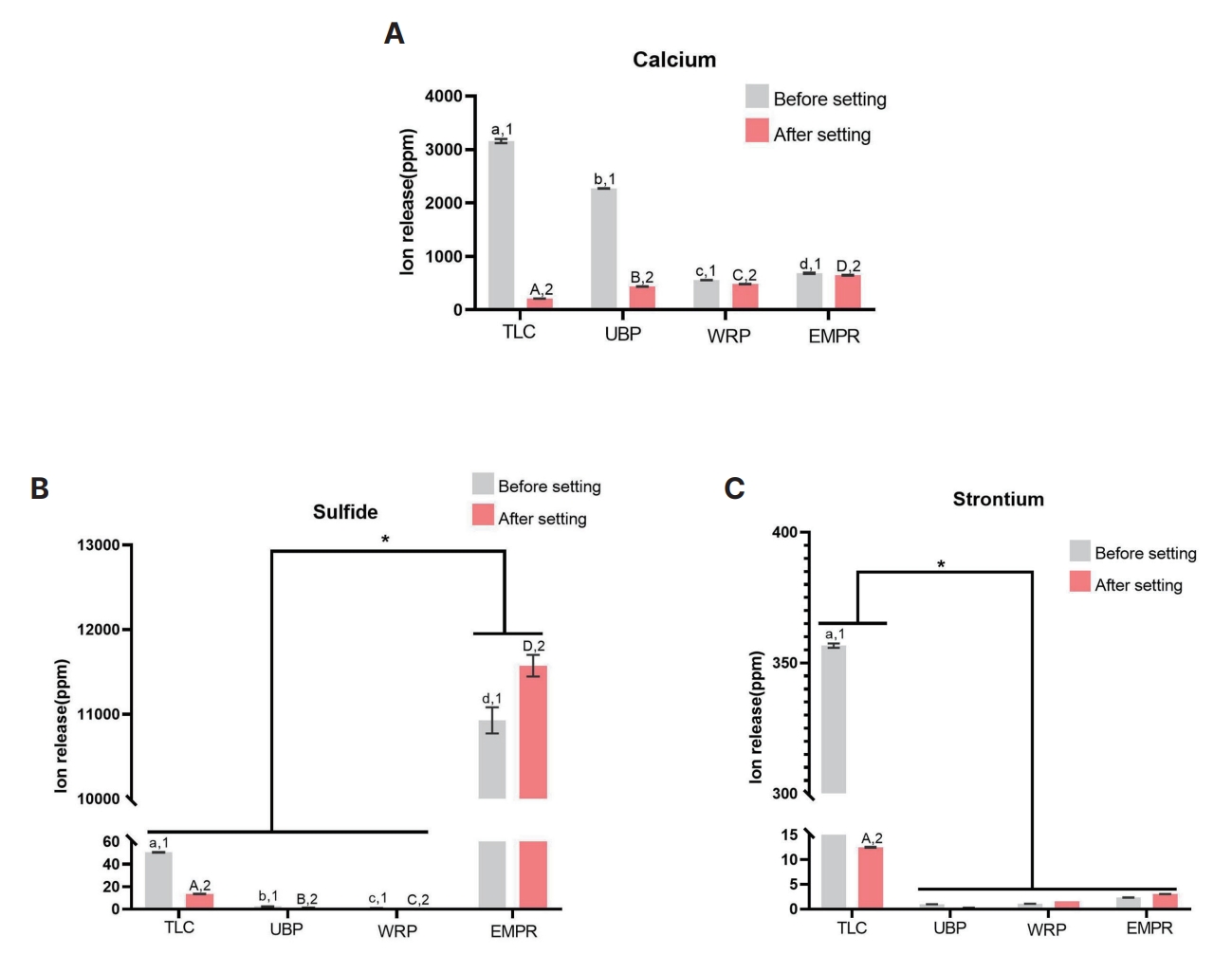
Mean concentration of ions released after 3 days of exposition to all tested dental capping materials. The lowercase letters indicate statistically significant differences (p < 0.05) between the before setting groups. The different uppercase letters indicate statistically significant differences between the after setting groups (p < 0.05) (n = 3). (A) Calcium ion, (B) Sulfide ion. Asterisks (*) indicate statistically significant differences between Endocem MTA premixed regular and the other materials, (C) Strontium ion. Asterisks (*) indicate statistically significant differences between TLC and other materials.
TLC = TheraCal LC®, UBP = Ultra-BlendTM plus, WRP = Well-RootTM PT, EMPR = Endocem MTA® premixed regular, n= number of measurements.
3. Solubility
Fig. 6 and Table 3 show the solubility test results after 24 hours and 1 week. Before setting, TLC had the highest mean solubility value after 1 and 7 days (p < 0.05). Before and after setting, EMPR had the lowest mean solubility value after 1 and 7 days (p < 0.05). The solubility value was significantly higher before setting than after setting in all groups (p < 0.05). In particular, TLC and UBP had significant differences in solubility value before and after setting (p < 0.05). After setting, the solubility values of all materials except UBP and EMPR after 7 days was significantly higher than those after 1 day (p < 0.05).

Mean solubility values of all tested dental capping materials after 1 and 7 days of exposition. The lowercase letters indicate statistically significant differences (p < 0.05) between the before setting groups. The uppercase letters indicate statistically significant differences between the after setting group (p < 0.05). The other numbers indicate statistically significant differences (p < 0.05) between each material before and after setting. Asterisks (*) shows statistically significant differences (p < 0.05) after 1 and 7 days (n = 3). (A) After 1 day, (B) After 7 days.
TLC = TheraCal LC®, UBP = Ultra-BlendTM plus, WRP = Well-RootTM PT, EMPR = Endocem MTA® premixed regular, n= number of measurements.
Discussion
Pulp capping materials play an essential role in successfully regenerating the dentin-pulp complex[34,35]. For a successful vital pulp treatment, these materials should have the following properties: (1) antibacterial activity (2) good biocompatibility (3) calcium ion releasing ability and (4) pulp cell damage to initiate immune process[36-38]. Several pulp capping materials including premixed MTAs, which can overcome the limitations of MTA, have been developed. Several studies have assessed the chemical properties of existing pulp capping materials after setting[31,38-41]. Some have compared premixed type and conventional MTAs. Results showed that premixed type MTAs have a higher calcium release capability and pH than conventional MTAs[42-44]. However, premixed type and conventional materials have similar cytotoxic risks and antimicrobial properties[45]. To the best of our knowledge, there are no previous studies comparing the 4 types of pulp capping materials before and after setting, and only few have assessed premixed MTAs. In this study, the chemical properties of 4 types of pulp capping materials were compared before and after setting to determine the properties of premixed MTAs and to assess the risk of toxicity in an incomplete setting.
In this report, all 4 materials had an alkaline pH after setting, and an alkaline pH has antibacterial activities and mineralization effects[6,27,46-48]. The pH of EMPR and WRP increased over time before and after setting. In particular, the pH increased evidently after 24 hours. This result is in accordance with that of previous studies about MTA[26,39,40,49]. According to Gandolfi et al. and Santos et al.[39,48], the effect of the hydroxyl group is released via the hydration of calcium silicate particles during setting. Moreover, after setting, the pH of resinmodified materials decreased over time, with a significant reduction within 12 hours. According to Yamamoto et al.[50], the resin matrix of TLC inhibits ion exchange and the hydration of calcium silicate components.
Previous studies have found that calcium, sulfide, and strontium ions at appropriate concentrations have antibacterial effects and can promote cell differentiation[51-54]. In particular, calcium ions are essential for the differentiation and mineralization of pulp cells and for the regulation of pulp calcification[30,38,55]. These ions are more likely to be detected in premixed MTAs after setting. This outcome is expected because calcium ions are released via the hydration of calcium silicate particles such as hydroxide ions[39,48]. EMPR contains calcium sulfate and dimethyl sulfoxide. Hence, it releases a high concentration of sulfide ion before and after setting. Moreover, it can have a high antibacterial effect. Relatively high concentrations of strontium ions were detected in a TLC before setting, which is attributed to the matrix of TLC containing strontium.
High solubility leads to loss of pulp capping materials and bacterial infection, which result in permanent sealing failure[5,41]. Low solubility is essential for pulp capping materials[2,31]. Previous studies have reported that EMPR has a high washout resistance and high mechanical strength because of its anti-washout ingredients such as hydroxypropyl methylcellulose and dimethyl sulfoxide[16,40,56]. This is consistent with the low solubility of EMPR in this experiment. The solubility value of WRP is relatively high, not because it has low washout resistance, but because it uses polyethylene and polypropylene glycols as solvents. According to ISO 6876[33], the solubility test is performed by drying water, and glycol that is not completely dried remains. Moreover, solubility data can be overestimated because, in the presence of biological fluids, the dissolution of water-soluble components and its associated weight loss are lower than distilled water and calcium phosphate deposition on the surface and inside of the tooth[38,41,57].
Moreover, resin-modified materials, particularly in TLC, had an irregular pH over time and a higher concentration of ion released and solubility value before setting than after setting. Additionally, there were similar differences in the pH of WRP and EMPR before and after setting. This result might be attributed to the fact that premixed MTAs can be set with moisture over time. Further, the resin-modified materials can be developed via photopolymerization. Hence, unpolymerized resin monomers may remain in resin-modified materials before setting.
WRP and EMPR had a higher pH and concentration of calcium ions released than TLC and UBP. In particular, EMPR released a high concentration of sulfide ions, and it had a low solubility value. In addition, in resin-modified materials, adequate light curing is important.
Based on the study results, WRP and EMPR are suitable pulp capping materials. However, this study had several limitations. First, the experiment was conducted in vitro, and no direct comparisons of pulp toxicity were performed. Second, the number of specimens was insufficient. Third, further studies should be performed to identify the interaction among pH, ion concentration, and solubility value. Finally, other chemical-mechanical tests, which can be used to evaluate cytotoxicity, water sorption, setting time, compressive strength, and radiopacity, should be conducted.
Conclusion
Recently, premixed MTAs have been developed. In this study, all materials had an alkaline pH and a low solubility value and could release various concentrations of ions. WRP and EMPR had a high pH, and their pH increases with time. Moreover, the concentration of calcium ions was evaluated. EMPR released a large concentration of sulfide ion and it had a low solubility value.
Resin-modified materials are commonly applied to deep cavities and incomplete curing can easily occur. Before setting, TLC and UBP had an extremely high ion release and solubility value, and their pH was extremely irregular. Curing should be performed accurately according to the manufacturer’s instructions.
Acknowledgements
This study was supported through the Research-Focused Department Promotion Project as a part of the University Innovation Support Program for Dankook University in 2021.
Notes
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Funding information
This research was funded by the Department of Dentistry (Pediatric dentistry) supported through the ResearchFocused Department Promotion Project as a part of the University Innovation Support Program for Dankook University in 2021.