불화은과 다이플루오르실란 함유 바니쉬의 Streptococcus mutans에 대한 항균 효과
Antibacterial Effects of Silver Fluoride and Difluorosilane-based Varnish on Streptococcus mutans
Article information
Abstract
연구의 목적은 2종의 액상형 불소 제재의 Streptococcus mutans에 대한 항균 효과를 평가하는 것이다. 불화은(AgF, 1제)과 요오드화칼륨(KI, 2제)로 구성된 Riva star aqua™ (SDI)와 Fluor protector® (FP; Ivoclar Vivadent)를 실험군에 사용하였다. 실험군은 4개 의 군으로 구분하였다: AgF, KI, AgF + KI, FP. 양성 대조군(PC)에는 ampicillin을 사용 하였고, 음성 대조군(NC)에는 아무런 처치도 시행하지 않았다. 각 군을 사용한 용액의 양 에 따라 30과 50 µL로 다시 분류하였고, 평판 도말된 S. mutans에 적용하였다. 이후 억제 대의 직경을 측정하였다. PC와 AgF는 모든 재료에 비해 큰 직경을 보였고(p < 0.05), AgF 는 50 µL군에서 PC와 유의한 차이가 없었다(p > 0.05). FP는 30 µL군에서 AgF + KI 보 다 큰 직경을 보였다(p = 0.009). KI는 NC와 유의한 차이가 없었다(p > 0.05). 불화은은 S. mutans에 대해 ampicillin과 유사한 항균효과를 보였으며, FP보다 더 뛰어남을 확인 하였다.
Trans Abstract
The purpose of this study is to evaluate the antibacterial effects of two liquid fluoride materials on Streptococcus mutans (S. mutans). Riva star aqua™ (SDI, Bayswater, Australia), which consists of silver fluoride (AgF, step 1) and potassium iodide (KI, step 2), and Fluor protector® (FP; Ivoclar Vivadent, Liechtenstein) were used for experimental groups. Experimental groups were divided into 4 groups : AgF, KI, AgF + KI and FP. For the positive control (PC) group, ampicillin was used, and the negative control group (NC) did not receive any additional treatment. Each group was divided into 30 and 50 µL groups by volume of liquid and applied to flat-coated S. mutans. The diameter of the zone of inhibition was measured. The PC and AgF groups showed larger diameters than other materials (p < 0.05), and the AgF group showed no significant difference from the PC group in the 50 µL group (p > 0.05). The FP group showed larger diameters than the AgF + KI group in the 30 µL (p = 0.009). The KI group did not show significant difference from the NC group (p > 0.05). AgF is comparable to ampicillin in antibacterial effects on S. mutans, and better than FP.
Introduction
Various types of microorganisms in the mouth form dental biofilms when they are placed on the surface of teeth and mucous membranes, causing chronic diseases such as dental caries and periodontitis. Acids produced as a result of microbial metabolism in dental biofilms acidify the oral cavity environment, resulting in demineralization of the tooth surface and dental caries[1,2].
Although there are various types of microorganisms that are considered the main causative agents of dental caries in the oral cavity, Streptococcus mutans (S. mutans) plays a major role in the initiation of tooth caries on enamel and tooth root surfaces[3]. S. mutans was first identified in human tooth cavities in 1924 and has excellent acid production, excellent viability in acidic environments, and surface antigen I, II, and can synthesize water-insoluble glucans that can be attached to tooth surfaces and other bacteria[3,4].
Fluoride, which is used in dentistry to prevent and suppress dental caries, is used in the form of tablets, syrups, foams, gargles, toothpastes, and varnish. In the past, the prevailing idea was that fluoride acts during tooth development to strengthen teeth, but in recent years, as local caries prevention mechanisms have been clarified after tooth eruption, suppression of demineralization, acceleration of remineralization, and inhibition of bacterial activity in the mouth are thought to be the main effects of tooth caries prevention[5].
Among the various fluoride materials, Fluor protector® (FP; Ivoclar Vivadent, Schaan, Liechtenstein), a volatile ethanol-based liquid varnish, is a product with a fluoride concentration of 1,000 ppm containing 0.9% difluorosilane, which is characterized by lower viscosity and higher flowability than resin varnish[6]. Another liquid fluoride material, Riva Star™ (SDI, Bayswater, Australia), is made of silver diamine fluoride (SDF), which was approved by the Food and Drug Administration in 2014 for dental hypersensitivity treatment and is used worldwide to prevent and stop dental caries[7]. Several studies have already demonstrated the re-mineralization and antibacterial effects of FP and SDF, consisting of silver, fluoride, and ammonia. Two-step products are also available for further application of potassium iodide (KI) to improve tooth discoloration by silver ions, which is considered a fatal side effect of SDF[7-9].
Recently, water-based silver fluoride (AgF) (Riva Star Aqua™, SDI) has been introduced to overcome the irritation of gingival tissue and the unpleasant smell caused by the ammonia component of SDF. The effect of Riva Star™ in reducing the number of S. mutans has been proven[9], but studies applying AgF to S. mutans are still insufficient.
The purpose of this study is to assess the antibacterial effects of silver fluoride and difluorosilane-based varnish on S. mutans.
Materials and Methods
1. Bacterial culture and smearing
S. mutans (ATCC 25175) from cold storage were incubated for 18 hours under atmospheric conditions at a temperature of 37°C and 5% CO2. The medium used was brain heart infusion broth (BHI; Becton, Dickinson and Company [BD], Sparks, MD, USA), and 1.5% agar (BD Bacto™) were added and sterilized at 121°C for 15 minutes. In the concussion incubator, the OD600 value was maintained at 0.5, and flat coating was performed on a flat agar medium. The flat smearing method was performed in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) version 10.0.
2. Experimental materials
Two types of liquid fluorine varnish, FP and Riva Star Aqua™, were used. Riva Star Aqua™ used both AgF (step 1) and KI (step 2). The entire package was opened immediately before using a new, unopened product and was used in this experiment. Ampicillin (Sigma-Aldrich, Saint Louis, USA) was used as the positive control (PC).
3. Experimental design
AgF alone, KI alone, a mixture of AgF and KI, and Fluor protector® were used as the experimental groups. AgF and KI were mixed with the liquid at a ratio of 1:2, as recommended by the manufacturer, and then mixed uniformly. Ampicillin was used as a PC to compare the antibacterial effects. The negative control group (NC) did not receive any additional treatment. The experimental and control groups were divided into 30 μL and 50 μL groups, and each volume was applied to an 8-mm paper disc (Whatman, Maidstone, UK), closely adhered to a flat agar plate medium, and cultured for 24 hours. Each experiment was independently repeated using six agar plates for each volume[10].
4. Component analysis of AgF and FP
Combustion ion chromatography was performed using a Dionex Aquion™IC (Thermo Fisher Scientific) to determine the concentration of fluoride in AgF and FP. Inductively coupled plasma spectroscopy using 5100 ICP-OES (Agilent Technologies) was performed for AgF to determine the concentration of silver.
5. Antibacterial effects assessment
One investigator measured the diameter of the zone of inhibition around the paper disc using a micrometer (Mitutoyo, Kawasaki, Japan). It was measured based on the distance from the center of the zone to the outer edge of the zone, and the final diameter was calculated as twice the measured value. The same measurement was performed for all flat media, and the mean and standard deviation for each experimental and control group were calculated.
6. Statistical analysis
Two-way analysis of variance (two-way ANOVA) and the Bonferroni post-hoc tests were performed to compare the types and volumes of the liquids (α = 0.05). All statistical analyses used SPSS 26.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
1. The diameter of the zone of inhibition by liquid type
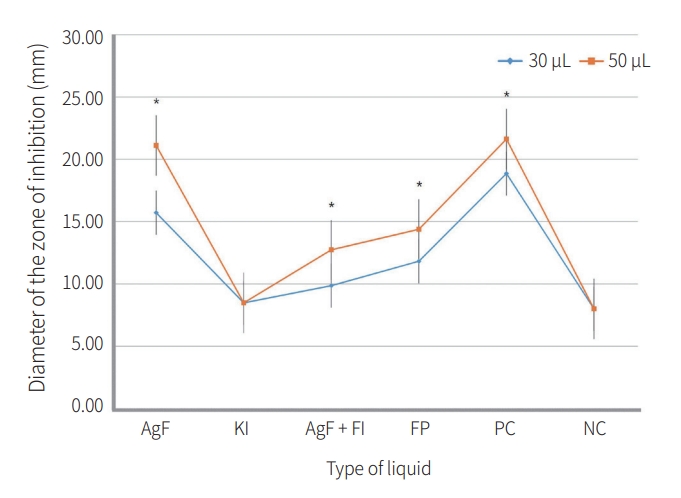
In the 30 μL group, the PC group showed larger diameters than the AgF group, and the PC and AgF groups showed larger diameters than all other materials (p < 0.05). The FP group had larger diameters than the AgF + KI, KI, and NC groups (p < 0.05). The AgF + KI group was not significantly different from the KI group (p = 0.196), and showed a significantly larger diameter than the NC group (p < 0.05). In the 50 μL group, the PC and AgF groups showed larger values than all other materials (p< 0.05), but there was no significant difference between the two materials (p > 0.05). The FP group was not significantly different from the AgF + KI group (p > 0.05), and showed significantly larger diameters than the KI and NC groups (p < 0.05). The AgF + KI group had significantly larger diameters than the KI and NC groups (p < 0.05, Table 1).
2. Diameter of zone of inhibition by liquid volume
For all materials except the KI and NC groups, the 50 μ L group showed larger diameters than the 30 μL group (p < 0.05, Fig 1). The AgF group did not significantly differ from the PC group in the 50 μL group (p > 0.05, Table 1).
3. Component analysis of AgF and FP
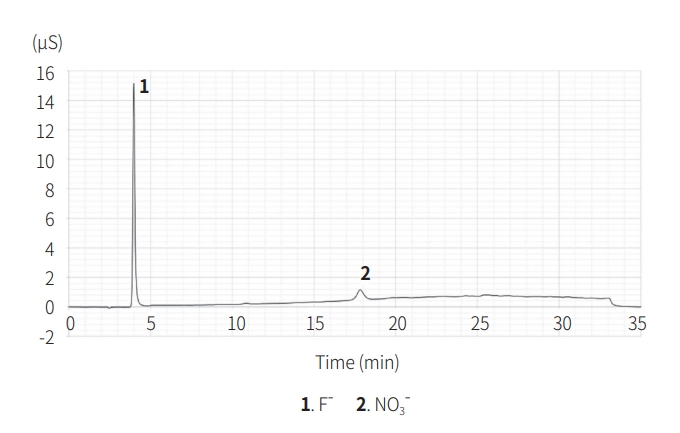
After the combustion ion chromatography analysis, the anions identified in AgF were F- (42,429 ppm), NO- (20,357 ppm) (Fig. 2), and those in FP were F- (662 ppm). Inductively coupled plasma spectroscopy analysis confirmed that the Ag content of AgF was 266,477 ppm, which was 26.65% of the total content. Therefore, approximately 30.89% of the silver fluoride from Step 1 was confirmed.
Discussions
The traditional method of managing dental caries was to repair damaged teeth and return them to their normal shape and function. However, as this is only a treatment for the consequences of tooth caries and not a fundamental solution, an approach to treatment that is more preventive than in the past was required[11]. As a result, non-invasive methods of tooth caries management, such as individual caries risk assessment, identification of bacterial organisms through saliva tests, oral pH management, and fluoride application, have become the main trends[11,12].
The first fluoride varnish was Duraphat®, which was developed in 1964 with 5% NaF as the main ingredient. It is applied to the surface of the tooth to suppress the disease, prevent the progression of tooth decay, and is effective in alleviating dental hypersensitivity[7]. Its main advantages include the continuous emission of fluoride ions and convenience of application, and it can be used even in situations where patient cooperation is relatively difficult[6,13]. In the 1970s, liquid fluoride varnish made of compounds like difluorosilane and SDF was created; while these varnishes were characterized by low viscosity, there were problems with their unique taste or the discoloration of teeth and tissues[8,14]. Recently, a water-based product that eliminates the ammonia odor of SDF has been developed, and there have been attempts to improve tooth staining with the addition of KI[9].
In this study, AgF (step 1) showed significantly higher antibacterial effects than all other materials, except for ampicillin. This is because silver, a fundamental component of Step 1, had a concentration of 266,477 ppm through inductively coupled plasma spectrometry. Silver can inhibit microbial growth in two ways. First, it hinders the electron transport system of cells, thereby restricting metabolic activity. Second, Ag+ is a positively charged ion that combines with the negative electric charge of the surface of cells or DNA, destroying the cell membrane and causing mutations. Furthermore, it reduces biofilm formation by microorganisms on behalf of calcium ions[15]. The AgF group showed increased diameters to the extent that there was no significant difference from ampicillin in the 50 μL group compared to the 30 μL group. Considering that one drop indicated by the manufacturer is 50 μL[16], it is considered that the antibacterial effect of AgF on S. mutans will be better according to the manufacturer’s instructions.
In the case of KI (step 2), no antibacterial effects were observed. In other studies on biofilm formation by S. mutans, no antibacterial effect was observed by KI alone[17]. When AgF and KI were mixed and applied, the degree of suppression was smaller than that of AgF alone. According to an in vivo study by Abdullah et al.[18], the SDF alone and SDF followed by KI did not show a significant difference in antibacterial efficacy. However, to quantify the volume of liquid of each group, AgF was mixed with KI in this study, precipitating silver ions into white silver iodide prior to application. This indicates that reducing potential silver ions by forming silver sediments before application to microorganisms can hinder the antibacterial ability of AgF. When applying to the oral cavity of an actual patient following a manufacturer’s instructions, the AgF and KI are used in a 1:2 ratio without mixing the two liquids. It is important to know that when the instructions are not followed, the KI used to improve tooth discoloration may interfere with the antibacterial activity of AgF on S. mutans in clinical environments.
FP confirmed some of the antibacterial effects by forming a transparent inhibition zone around the disc, which was less than that of the PC and AgF groups. FP is a transparent liquid product containing polyurethane-based difluorosilane and 1,000 ppm of fluoride[6]. The fluoride concentration of FP confirmed in this experiment was 662 ppm, which is somewhat different from the known data[6,8]. Fluoride temporarily increases the hydrogen ion permeability of microorganisms, preventing metabolism from occurring smoothly[15]. According to a study by Erdem et al.[8], the silane in FP is responsible for its antibacterial activity against S. mutans, despite its relatively low fluoride concentration compared to other fluoride varnishes. They further showed that the addition of chlorhexidine-based gel products to FP has higher antimicrobial properties. However, further studies are needed to determine whether it is clinically meaningful to control local cariogenic organisms by adding artificial substances to improve the antimicrobial effect.
Analysis by combustion ion chromatography revealed that AgF contained approximately 2% nitrate, a significant decrease from the previous value of 15% or more[19]. Ammonia molecules in SDF are used to stabilize high concentrations of liquid, bind to silver ions, and transfer them to diamine silver[15,20]. As AgF alone can confirm an antibacterial effect equivalent to ampicillin, ammonia may be excluded from the perspective of antibacterial ability and thus remove the unpleasant smell.
Recently, studies have been conducted to apply nanoscience to fluoride products to solve the discoloration caused by silver ions away from two-step products. Nanosilver fluoride (NSF), a water-based mixture of silver nanoparticles and NaF varnish, is designed to complement the shortcomings of conventional SDF while also having similar tooth caries prevention and antibacterial activity[21]. The main advantage is that silver nanoparticles can increase the antibacterial effect by significantly increasing the surface area that can be exposed to microbial communities and can be safely used in anterior dentition with no concern for discoloration. Owing to its low pH and high biocompatibility, it does not irritate oral soft tissues[22]. If a comparative study is conducted on the antibacterial effect of the two water-based materials in the future, the effects of silver ions and silver nanoparticles on S. mutans can be compared.
The limitations of this study are as follows: First, additional studies are needed to confirm multiple applications to various microorganisms and long-term antibacterial durability as this study was only observed and measured for 24 hours after applying to a single species, S. mutans. And because this study is on the pilot stage of Riva star aqua™, more experiments are needed to determine the minimum inhibitory concentration and the minimum bactericidal concentration. Second, it will be helpful to understand the clinical implications by creating similar environments to the oral cavity, following the manufacturer’s instruction step, and applying materials to the tooth or a similar ingredient, like hydroxyapatite[23,24]. Third, according to the manufacturer’s explanation[25], the AgF content of Riva star aqua™, which was thought to be the same at 38% as SDF, was confirmed to be 31% in the study. Errors in the sample analysis process should be considered, and if there is a change in the concentration of AgF, the difference in the antibacterial ability remains a research task. In addition, as AgF does not yet have international guidelines on its application cycle, frequency, and method of use, additional research is needed in addition to the manufacturer’s instructions.
Conclusions
This study compared the antibacterial effects of two types of liquid fluoride materials, Riva Star Aqua™ and Fluor Protector®, on S. mutans by setting groups according to solution type and volume.
The AgF group showed better antibacterial effects than the KI, AgF + KI, and FP groups regardless of the volume of liquid. At a dose of 50 μL, AgF exhibited an antibacterial effect similar to that of ampicillin. The antibacterial effect could not be confirmed using KI alone; FP and AgF + KI had antibacterial effects, but they did not match those of AgF and ampicillin.
From the above results, the AgF is comparable to ampicillin in antibacterial effects on S. mutans and better than FP. Further research on its efficacy in the clinical setting is needed.
Notes
Conflict of Interest
The authors have no potential conflicts of interest to disclose.