양산시 거주 13-15세 학생의 치아침식증 유병율과 위험요소
A Survey on the Prevalence and Risk Indicators of Dental Erosion among 13-15 Year Old Adolescents in Yangsan, Korea
Article information
Abstract
최근 세계적으로 치아침식증에 대한 관심이 급증하고 인식의 변화가 나타나 많은 연구보고가 있었다. 우리나라 국민건강 영양조사에 따르면 청소년기로 갈수록 1일 탄산음료 섭취량이 늘고 있는 추세이며, 이는 청소년들이 치아침식증에 취약할 수 있음을 시사한다. 하지만 우리나라에서는 이에 대한 인식이 아직 미진하고, 치과계에서도 연구적, 임상적 관심이 그리 높지 않은 실정이다. 본 연구는 양산시 청소년들의 영구치에 나타난 치아침식증의 유병율을 조사하고 설문지를 통하여 그 위험요인을 분석할 목적으로 시도되었다.
양산시에 거주하는 13-15세 중학생 1,371명을 대상으로 단일 검사자가 영구치의 치아침식증 상태를 조사하였다. 치아침식증의 평가기준으로는 Visual Erosion Dental Examination system을 사용하였다. 또한 설문지를 통하여 이들의 식이습관 및 구강위생상태와 치아침식증 간 유의성을 비교ㆍ분석하였다.
치아침식증은 676명(49.3%)에서 관찰되었고, 남성(46.4%)에 비해 여성(53.0%)이 더 높은 유병율을 보였다(p < 0.05). 상악(39.3%)에 비해 하악(43.0%)에서 더 높았으나(p < 0.05), 좌ㆍ우측 간에는 차이가 없었다. 구치에 비해 전치의 유병율 및 치아 당 평균점수가 높았다. 가당 및 무가당우유는 치아침식증에 영향을 미치는 반면(p < 0.05), 다른 음료, 식이섭취방법, 구강위생, 그리고 BMI의 영향은 미미했다(p > 0.05).
본 연구에서 나타난 유병율은 20~30% 내외인 다른 보고들에 비해 높았으며, 남성이 호발한다는 기존의 보고들과 달리 여성에서 유병율이 더 높았다.
Trans Abstract
It is a trend that carbonated drink intake among adolescents is increasing, which makes young people more vulnerable to dental erosion. However, in Korea, public knowledge about dental erosion is very insufficient. The aim of this study was to investigate the prevalence of dental erosion and to assess its risk indicators among 13-15 years old students in Yangsan, Korea.
A total of 1,371 adolescents were examined by one calibrated clinician. Dental erosion was assessed by using the Visual Erosion Dental Examination system. Correlation between their dietary habit, oral hygiene and dental erosion was assessed.
The data showed that 676 (49.3%) adolescents had dental erosion. The prevalence of dental erosion was significantly higher in females than in males. The prevalence of tooth erosion in mandible is higher than in maxilla. Dental erosion was generalized to develop mostly on anterior teeth, especially lateral incisor, however, the severity score was highest in canines. Following questionnaire analysis, dental erosion was significantly associated with milk and flavored milk. No other associations were detected.
The prevalence of dental erosion in this study is higher than those of previous reports. On the contrary to previously reported studies, the prevalence of dental erosion in females is higher than in males.
Ⅰ. Introduction
The damage to the structure of the dental hard tissue is the result of complex reactions of physical and chemical stimuli. One of the main causes of damage is the chemical stimulus by acid. Dental caries is the representative oral disease. This is the demineralization of teeth by acid and the destruction of internal organics damaging dental tissues [1]. Non-carious factors are: abrasion appeared by the friction of extrinsic substances, attrition caused by antagonist teeth, abfraction resulted by tensile and compressive forces, and erosion, which is gradually losing hard dental tissues and causing irreversible damage due to chemical reactions of acid or chelating materials [2]. Erosion is the biggest risk factor to loss of dental tissues among these non-carious risk factors [3].
World Health Organization (WHO) set up a goal until the year 2,000 to decrease the average index of people with permanent teeth that had experienced caries [4]. As a result, dental caries decreased steadily and still continue to decrease [5-7]. However, the prevalence of dental erosion has consistently increased especially in young ages [8-12]. This has brought a change in perception about dental erosion and increased awareness worldwide. The prevalence and severity of dental erosion clearly increased over the past few decades, and it is evidently worse especially in adolescents [13].
Dental erosion is a multifactorial disease caused by interactions of oral hygiene habits, lifestyle, health status, and eating habits [14]. Among these contributing factors, excessive consumption of acidic drinks and food is one of the significant factors causing dental erosion. There is an increasing tendency in the frequency and total amount of acidic drink and food intake due to changes in lifestyle, particularly in adolescence [15]. According to the sixth Korea National Health and Nutrition Examination Survey (KNHNES)(2013), adolescents’daily intake of carbonated drink show a growing trend, which means adolescents could be more vulnerable to dental erosion [16].
Dental erosion causes an irreversible loss of dental tissues, and requires extensive restorative treatment after reaching a certain stage. Detection as early as possible, is very critical to prevent such a progress [6]. It is also important to find the risk factors causing dental erosion. Although dental erosion has come in to the worldwide spotlight, it has not received much attention domestically. In fact, research and clinical interest of dentistry is not particularly high [17,18], especially in adolescents.
The purpose of this study is to research the prevalence of dental erosion, and to compare and analyze similarities of contributing factors related to this, such as eating habits, oral hygiene habits, food and beverages. This was done by surveying these factors appearing in middle school students (13-15 years old) who attend schools in Yangsan, Korea.
Ⅱ. Materials and Method
1. Subjects
The status of dental erosion was researched on 1,380 students (13-15 years old) from four different middle schools in Yangsan and the risk factors were evaluated through this survey. The questionnaire was done prior to performing oral examination. 1,371 students (response rate = 99.3%) became final survey subjects, except nine students who did not participate on the survey or return the questionnaire. Age and gender distribution of the survey are as followed (Table 1).
2. Examination method
1) Preliminary investigation
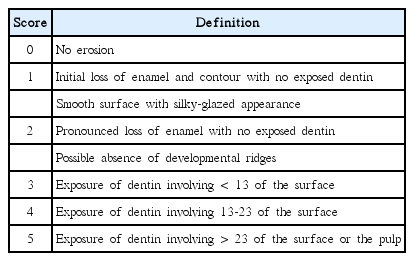
The evaluation training was conducted prior to an epidemiological survey by using clinical oral pictures of adolescents who are the same age range of the survey subjects. Visual Erosion Dental Examination (VEDE) system [19] was used as diagnostic criteria for dental erosion (Table 2). The oral pictures of 50 adolescents, which include mandibular and maxillary labial, buccal, occlusal, and lingual surfaces, were evaluated by the diagnostic criteria of this research.
2) Oral examination
The oral examination was performed, and the status of dental erosion was investigated and recorded by one trained examiner using headlights and a dental mirror in each school’s health unit. Food residue and plaque were removed by using gauze when necessary before the oral examination. Any retained primary teeth, missing permanent teeth, and extensive restoration teeth were excluded from the examination subject.
3. Survey method
1) Survey
The questionnaire including questions about eating habits, oral hygiene habits, and the obesity of adolescents, was designed to analyze the correlation between dental erosion and these factors. The obesity was evaluated by Body Mass Index (BMI). BMI is a person’s weight in kilograms divided by the square of height in meters. A high BMI can be an indicator of high body fatness [20]. The eating habits include frequency, time, and methods of various drink consumption. The oral hygiene habits include frequency, time, and techniques of brushing teeth. The question about vomiting symptoms which is an intrinsic factor causing dental erosion, was also added.
2) Data analysis
The mean and standard deviation were used for general characteristics of the subjects, and the difference between each group was analyzed by an independent t-test. A chi-squared test was used to determine whether or not there is an association with the risk factors. The correlation between drink consumption habits and dental erosion was tested by binary logistic regression analysis. SPSS 13.0 (SPSS Inc., U.S.A.) for Windows was used for statistical analysis. All statistics’significance level was set to be 0.05.
4. Ethical considerations
Deliberation exemption was approved to this study, which is in accordance with the examination of the bioethics committee of the Pusan National University Dental Hospital (PNUDH-2013-019).
Ⅲ. Results
1. The prevalence of dental erosion
The prevalence of dental erosion about the survey subjects of the 1,371 adolescents is shown in Table 3. At least one tooth with dental erosion was observed in 676 out of 1,371 (49.3%) adolescents survey subject, 355 out of 765 males (46.4%) and 321 out of 606 females (53.0%), showing that it was found significantly more in females than in males. The prevalence of the age of the patient in the case of 13 year olds was 199 out of 316 people (63.0%). In the case of 14 year olds, it was 295 out of 498 people (59.2%), and in the case of 15 year olds, it was 182 out of 557 people (32.7%). Studies showed that the prevalence of dental erosion was statistically low as the age increased.
The comparison between maxilla and mandible about the prevalence distribution of dental erosion shows that it is significantly higher in the mandible, which was 590 people (43.0%), and 539 people (39.3%) in the case of the maxilla (p < 0.05, Table 4). When the left and right sides were compared by using the midline of maxilla and mandible as its standard, it was 675 out of 1,371 people (49.2%) in case of the left and 673 (49.1%) in case of the right, but it did not show a significant difference.
2. Distribution and severity of dental erosion
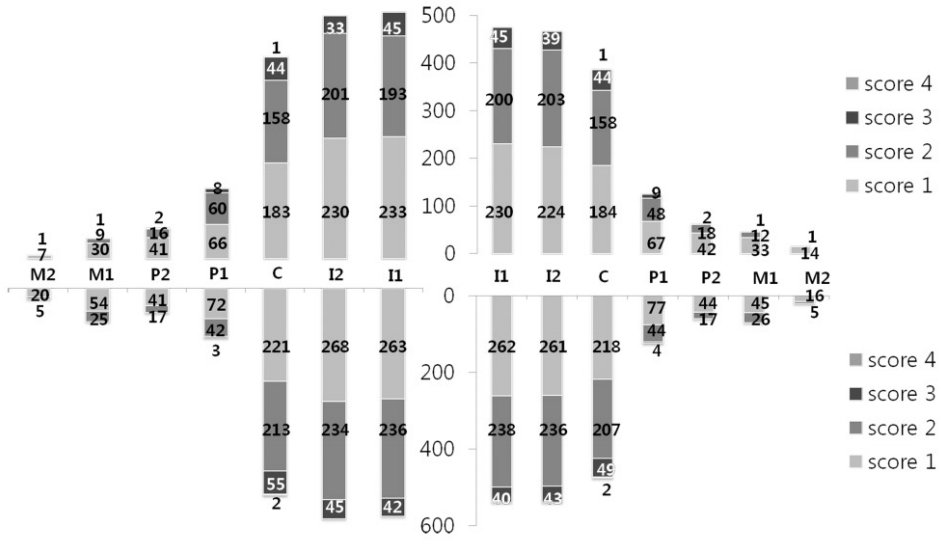
The result of distribution and severity from dental erosion on each tooth showed that mandibular right lateral incisor had the highest erosion rates of 39.9%, and the lowest erosion rates on the maxillary right second molar (Table 5). The severity of dental erosion using VEDE score was indicated with the average value after measuring severities of each tooth. The severity was the highest on the mandibular right canine and the lowest on the maxillary left second molar (Table 6). The distribution and severity of dental erosional lesions of each tooth is shown in Fig. 1 and the most subjects to show dental erosion have the scores of 1 and 2.
3. The relationship between dental erosion and eating habits of adolescents
1) Frequency of consuming carbonated drinks, fruit juices, milk, and fruit
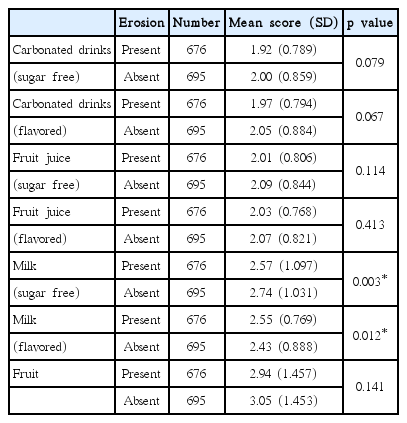
Sugar free milk intake frequency was lower in the erosion group but it was higher for flavored milk (Table 7). Flavored milk is a sweetened dairy drink made with milk, sugar, colorings and artificial or natural flavorings. It was revealed that there is a significant relationship between the frequency of milk and flavored milk intake and dental erosion (p < 0.05). The association of dental erosion with other beverages was significantly lower (p > 0.05).
2) The methods of consuming carbonated drinks, fruit juice, milk, and fruits.
There was one investigation whether or not a straw was used as a way of consuming, and another investigation whether or not drink or food was consumed before bedtime (Table 8). The group that used a straw showed the higher rate of dental erosion, but there was no statistical correlation (p > 0.05).
4. The relationship between BMI of adolescents and dental erosion.
There was no statistically significant correlation between dental erosion and BMI (Table 9). Commonly accepted BMI ranges are underweight : under 18.5, normal weight : 18.5 to 25, overweight : 25 to 30, obese : over 3020).
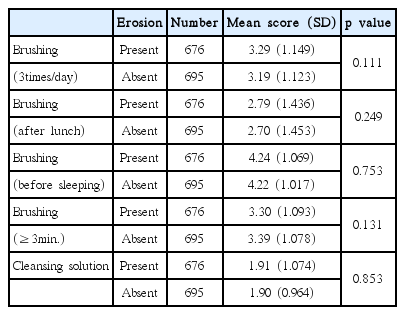
5. The relationship between oral hygiene habits and dental erosion
Regarding the relationship between oral hygiene habits and dental erosion, there was no statistical correlation (Table 10).
Ⅳ. Discussion
Adolescence is a stage gets into the permanent dentition, and if exposed to oral disease caused by neglected oral hygiene at this period, there would have an adverse effect on oral health. Dugmore and Rock [15] conducted a survey of dental erosion for the age of 12 and two years later in the group of 1,753 adolescents. At the age of 12, 59.7% of the adolescents had an erosion lesion and 2.7% of them had dentin exposure. Two years later, dentin exposure was increased by 8.9% in the dental erosion test, reporting that the severity of dental erosion was significantly higher after two years. Severity of dental erosion was increased over time which showed a newly created pattern. This suggests that it could fail to minimize damage to the permanent dentition unless the preventive treatment is performed by early detection of dental erosion. On the basis of these reasons, this study was conducted to investigate the prevalence and risk factors of dental erosion in the permanent dentition.
In the initial stage of dental erosion, it is hard to make a diagnosis of erosion because it appears without clinical symptoms of pain or sensitivity. Wang and Lussi [21] said that it is important to determine by using the clinical characteristics of dental erosion because there is no device can accurately measure dental erosion and its progression. Larsen et al. [22] reported that a clinician is hard to distinguish between healthy enamel and enamel lesions.
The clinical characteristics of dental erosion are as follows [21]: when affected in an enamel, tooth surface is smooth and glossy, and the lesion is often located in the crown side from the cemento-enamel junction. This is because health enamel margin is appeared in the gingiva where gingival cervical fluid and plaque exist. Plaque blocks the acid and gingival cervical fluid neutralize it. When the occlusal erosion is progressed, the position of the restoration is higher than the height of the tooth surface of the adjacent tooth. In the severe case, the entire occlusal morphology is lost which can lead to dentin and pulp exposure [23].
The dental erosion should be distinguished from attrition [24]. If an attrition is an exclusive cause, it should be appeared in the occlusal contact point and does not appear from the labial and lingual side of teeth but can occur from the lateral and anterior movement of the mandible. The morphological features of an attrition are shiny, flat, and sharp edge. Also, attrition appears consistently in the antagonist teeth with the teeth that the attrition appears. If attrition appears in the maxillary anterior teeth, attrition not appeared in the mandibular anterior teeth is not possible. In case of dental erosion, it can not be appeared in the occlusal contact part like labial and lingual surface of teeth.
The cupping phenomenon is shown rather than showing morphologically smooth, polished, and flat surface of teeth and the cusp and groove are round rather than sharp. It does not appear in the maxillary and mandibular incisor together, but each appears. This appears in the person who has an anterior open bite, and when trying anterior transposition it can determine the type of abnormalities in the anterior teeth although having no contact on the anterior teeth, which can be thought of as tooth erosion caused by acid. It was difficult to distinguish among attrition, abrasion, and dental erosion accurately. This is because there exists a possibility that attrition, abrasion, and dental erosion often occur at the same time. Because teeth become weak followed by demineralized enamel layer cased by dental erosion, it becomes more vulnerable to the attrition and abrasion.
In the past, most indices to clinically diagnose dental erosion were to modify the Eccles index [25] and Smith and knight index [26]. Many indices were developed afterwards but dental erosion index universally agreed does not exist so far [27]. The ideal characteristics of dental erosion proposed by Bardsley [28] are as follows. It should be simply usable and understandable. Also, the criteria of scores should be clear and reproducible. When investigating the cause, it should be useful in the prevention and monitoring. Lastly, it should be able to be used as an epidemiologic and clinical tool. The two kinds of index most commonly used to assess dental erosion are Visual Erosion Dental Examination (VEDE) and Basic Erosive Wear Examination (BEWE) [29]. VEDE and BEWE are widely used because of their high reproducibility and appropriateness, but they are not free from error. VEDE system and BEWE system are similar in many ways. But VEDE system conducts a test without distinction of tooth surface and divides the affected part into a two - step and the range of affected dentin into 1/3 unit. Thus when using the VEDE system it tends to increase index score of dental erosion. In the early stage of enamel erosion, the VEDE system which departmentalizes affected enamel displays easier tendency to commit errors than BEWE system [19,30]. In this study, the VEDE system was applied in the initial state mostly limited to the enamel in order to separate the dental erosion affected to the enamel in detail. It was reported that distinguishing the early enamel lesion from the health enamel exists the difficulty, but they tried to overcome it through pretraining [31].
For the subject of this investigation, 13-15 year-old middle school student of 1,371 people, the prevalence of dental erosion was about 676 people (49.3%). This was relatively higher than other research papers which has used the same dental erosion index targeting a similar age. Arnadottir et al. [32] reported that the prevalence of dental erosion for 1,507 people whose age is 12 and 15 year old living in Iceland was 349 people (23.1%) and Mulic et al. [33] reported that the prevalence of dental erosion for 1,456 people of 18 year-old living in Norway was 554 people (38.0%). There exist many studies that has mentioned dental erosion is observed more significantly in males than in females [33-35]. This is because females have a significantly thicker enamel than males, while males have stronger muscular strength and masticatory muscle, and more consumption of carbonated drinks than femalesb[36]. However, this study appeared to be significantly higher in females and males (p < 0.05) as a result of 355 out of 765 in case of men (46.4%) and 321 out of 606 in case of women (53.0%). Bere et al. [37] reported that female has a higher tendency in the intake of fruits and juices. Certain research paper has reported that in one’s sleep, if the acidic product touches the tooth, dental erosion often occurs which is due to reduced saliva production. Also, the fact that female has lower salivary secretion than male could be another reason. Wang and Lussi [21] suggested that the flow rate and buffering capacity of saliva is one of the etiological factors, so it is important to determine them.
It was reported that the consumption of acidic beverages had a significant contribution to the presence and progression of dental erosion increases [38]. However, this study has reported that the relationship between the dental erosion and the acidic beverage is not statistically significant, but male has higher intake number of the acidic beverage than female. Therefore, the link between milk intake and dental erosion was statistically significant. Amaechi et al. [39] and Gedalia et al. [12] have reported that intake of dairy products such as fresh milk remineralizes the softened tooth surfaces and this shows similar result with the study has reported that it would be used in situations such as erosion. According to Magalhães et al. [40], the fluorine-containing milk will prevent dental erosion and this effects will be increased at higher concentrations of fluoride. Also, in case of affected dentin by erosion, drinking the milk does not contain fluorine after the erosion will prevent erosion afterward.
The prevalence in relation to the subject’s age is 199 out of 316 people (63.0%) in case of 13 year olds, 295 out of 489 people (59.2%) in case of 14 year olds, and 182 out of 557 people (32.7%) in case of 15 year olds, which shows as age increases, the prevalence of dental erosion is statistically and significantly low. As age increases, the rate of dental erosion caused by consumption of fruit, flavored milk and acidic beverages such as sugar free carbonated drinks and fruit juice was low (p < 0.05).
Most of the research on dental erosion was mainly aimed at the maxillary anterior teeth and mandibular molars. However, these surveys do not provide an information about how the dental erosion appears on all dentition [41]. Becuase especially national survey of adolescents in this study was insufficient, this study researched on distribution and severity of dental erosion in all the permanent teeth. Most of dental erosion affected on dentin was incisal edge of incisor and canine, and the buccal surface of the maxillary and mandibular anterior teeth had higher tendency compared to posterior teeth [9]. In this study, the prevalence and severity of dental erosion is definitely higher in anterior teeth that in posterior teeth, and the comparison between maxilla and mandible about the prevalence of dental erosion shows that it is significantly higher in the mandible as 43.0% than in maxilla as 39.3%. Sognnaes et al. [42] pronounced that mandibular teeth showed a higher frequency of erosion-like lesions than the corresponding maxillary teeth (21.0% compared to 13.0%). The highest percentage of erosion-like lesions was found in mandibular incisors (28.0%). This study supports that the frequency of dental erosion was higher in mandibular teeth.
Recently, the attention on the influence of obesity problem of adolescents on oral disease is increasing, and so BMI using height and weight is used in order to investigate the correlation between the BMI of adolescents and dental erosion. Because obesity is caused by lifestyle, such as dietary habits rather than genetic or endocrine factors [41], there is a tendency to consume a large amount of the acidic food and beverage in case of obese patients. Hence, the research to investigate the correlation between obesity and dental erosion has been progressed [43]. It was reported that the prevalence of dental erosion was low in case of underweight and high in case of overweight and obesity compared to healthy people, but there was no significant difference in this study. McGuire et al. [44] reported that underweight adolescents had the lowest prevalence of dental erosion, however there was no significant difference among the groups showing the results that are similar with this study. Future studies examining the relationship between obesity and dental erosion are required to provide dentists the ability to say whether obesity could be one of the risk factors for dental erosion.
The result has shown no link between oral hygiene and dental erosion, but because the prevalence of dental erosion is significantly decreased in accordance with the oral hygiene condition by age (p < 0.05), other studies will be needed for this. The result about the correlation between dental erosion and milk intake has come, hence we need to further study in order to find out the component of milk that contributes on this result. Although findings of this study are particularly important to dentists for the purpose of diagnosing dental erosion at an early age, limitations of this study should be noted. This study was cross-sectional study which can only observe the current state when the changes appearing over time cannot be observed. Research on permanent tooth erosion is being constantly published, but because the research on primary tooth erosion is uncommon except for the research paper published by Yu et al. [18], more studies about it will be needed. Further research is also needed to find more significantly related risk factor.
Ⅴ. Conclusion
For the purpose of realizing the status of dental erosion for adolescents and analyzing and evaluating related risk factors, the clinical examination and the questionnaire were done for a total of 1,371 adolescents aged 13-15 years attending in certain middle schools in Yangsan. Results of analyzing the prevalence of dental erosion and relation between oral hygiene and dietary habits of teenagers were as follows. The data showed that 676 adolescents out of 1,371 had dental erosion (49.3%), and dental erosion was observed in 355 people out of 765 males (46.4%) and 321 people out of 606 females (46.4%), concluding that the prevalence of dental erosion was significantly higher in female than in male (p < 0.05). The prevalence of dental erosion in mandible (43.0%) is higher than in maxilla (39.3%) (p < 0.05). There was no significant difference between the prevalence of right and left sides in maxilla and that of mandible (p > 0.05). Comparing to the group of nondental erosion, the group of dental erosion was significantly high in flavored milk intake (p < 0.05), but low in sugar-free milk intake (p < 0.05). No other significant relation between dental erosion and the intake of other beverages, frequency of oral hygiene, and BMI was detected (p > 0.05). In conclusion, the prevalence of dental erosion as 49.3% for adolescents in Yangsan, Korea was higher than worldwide prevalence using VEDE scoring system. The prevalence of dental erosion in female was higher than in male, and the frequency of intake of flavored milk and sugar-free milk was investigated as a contributing factor of dental erosion.