레진강화형 글라스아이오노머의 초기 결합력과 타액오염 제거의 상관관계
Effect of Saliva Contamination Stage and Different Decontamination Procedures on Bonding Strength of Resin-Modified Glass Ionomer
Article information
Abstract
이 연구는 타액오염이 발생한 시기와 타액오염 제거 방법이 레진강화형 글라스아이오노머의 상아질에 대한 결합력에 미치는 영향에 대해 평가하고자 하였다.
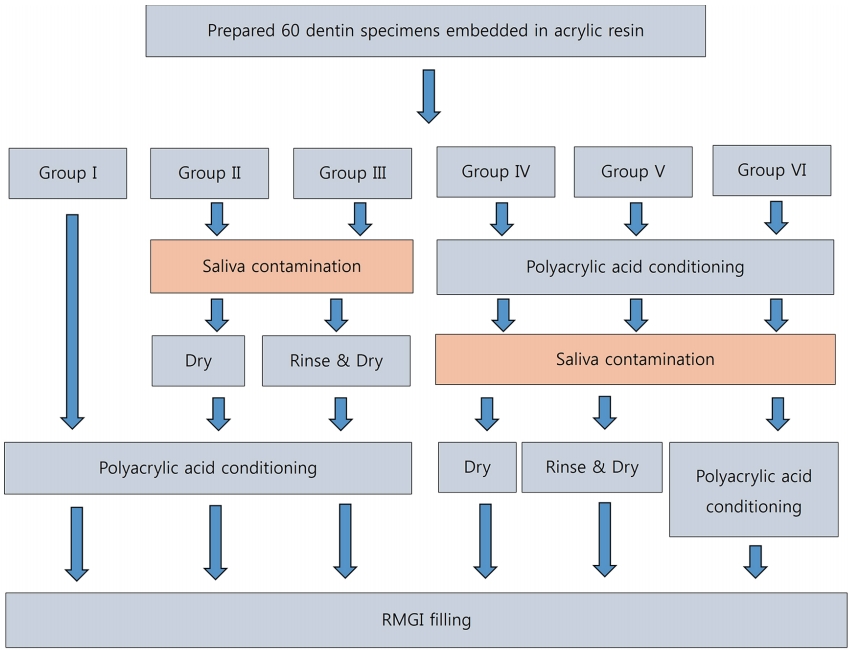
각 군당 10개씩 총 60개의 발거된 영구치 상아질 표면을 노출시켜 아크릴 레진에 매몰하였다. I군은 대조군으로 폴리아크릴산(PAA)으로 산처리만 시행하였다. II, III군은 PAA 산처리 전 타액오염을 시켰고 IV, V, VI군은 PAA 산처리 후 타액오염을 시켰다. 타액오염 후 II군과 IV군은 건조하였고 III군과 V군은 수세 후 건조하였으며 VI군은 추가적으로 PAA 산처리하였다. 그 후 레진강화형 글라스아이오노머를 충전하였다. 전단결합강도는 만능 재료 시험기로 측정하였고 파절 양상은 주사전사현미경으로 관찰하였다.
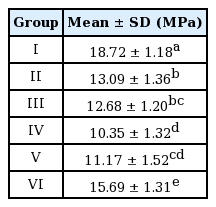
대조군인 I군이 유의하게 가장 높은 전단결합강도를 보였다(p = 0.001). 타액오염을 시행한 군들 중에서는 VI군이 유의하게 높은 전단강도를 보였다(p = 0.001). 파절양상은 군간에 유의한 차이를 보이지 않았다(p = 0.729).
타액오염은 발생한 시기와 상관없이 레진강화형 글라스아이오노머의 상아질에 대한 결합력을 유의하게 저하시켰다(p = 0.001). 수세와 건조만으로는 전단결합강도를 회복하지 못했다. PAA 산처리 후 타액오염이 발생한 경우 추가적인 PAA 산처리가 전단결합강도를 유의하게 향상시켰다(p = 0.001).
Trans Abstract
The purpose of this study was to compare the bond strength of resin-modified glass ionomer (RMGI) to dentin with saliva contamination at different stages and using different decontamination procedures
Extracted human permanent molars were embedded onto acrylic resin with the dentin surface exposed. Group I was a control group that was conditioned with polyacrylic acid (PAA). Groups II and III were contaminated with saliva before PAA conditioning and Groups IV, V, and VI were contaminated with saliva after PAA conditioning. After saliva contamination, Groups II and IV were dried, Groups III and V were rinsed and dried, and Group VI was additionally conditioned with PAA. After surface treatment, the dentin specimens were filled with RMGI.
Group I showed significantly higher bond strength than the other groups. Group VI showed a significantly higher bond strength than the other saliva contaminated groups. However, there were no significant differences in the failure mode between the different groups.
Saliva contamination impaired the bond strength of RMGI to dentin, regardless of when the saliva contamination occurred. Decontamination with washing and drying could not improve the shear bond strength of RMGIC. When saliva contamination occurred after PAA conditioning, additional PAA conditioning improved the shear bond strength.
Ⅰ. Introduction
Resin modified glass ionomer (RMGI) cements are widely used for class I and class II restorations in children and adolescents, as they have superior physical properties over conventional glass ionomer cements and the ability to release fluoride. The property of releasing fluoride makes it advantageous to use RMGI restorations in children with a high risk of tooth caries[1]. RMGI cements also have an additional use in temporary restoration for patients who are un-cooperative due to poor co-ordination skills and for those with systemic diseases where permanent treatment must be delayed until the patients are sufficiently stable[2].
Both pediatric patients and people with special needs are always at risk of saliva contamination during restorative treatment because of poor co-ordination skills and their oral conditions. The surface condition of the tooth before restoration affects the bond strength of the restoration. The saliva contamination of the tooth surface has adverse effects on the bond strength between the restorative material and the tooth surface[3,4].
Saliva contains salivary proteins, enzymes, microorganisms, food residues, and other organic substances[5,6]. If these remain on the tooth surface, they impair the bond strength between the restorative material and the tooth surface[3]. In order to ensure adequate bond strength of the restoration, it is necessary to clean the contaminated tooth surface; this may include treatments such as water rinsing or additional acid etching.
Although there have been studies on saliva contamination on bond strength of RMGI, no studies have investigated the bonding strength of RMGI according to saliva contamination stage.
The purpose of this study was to compare the bond strength of RMGI cements on dentin, based on saliva contamination at different stages and using different decontamination procedures.
Ⅱ. Materials and methods
1. Specimen preparation
This study was approved by the Institutional Review Board of Gangneung-Wonju National University Dental Hospital (IRB 2018-007).
60 human, non-carious, extracted permanent teeth were collected and stored in distilled water at 4.0℃ until use. Only molars with no wear defects, fracture lines, or cracks were included in this study. Soft tissues attached to the selected teeth, if any, were removed using a hand scaler.
A flat dentin surface parallel to the occlusal plane was obtained using a diamond cutting disk. The teeth were embedded on self-cure acrylic resin with only the crown portion visible. The tooth surface was made even using 220-grit silicon carbide abrasive paper and then polished with a 600-grit silicon carbide paper to standardize the dentin surface.
Details about the materials used in this study, namely the RMGI (Fuji II LC capsule, GC Corp., Tokyo, Japan) and polyacrylic acid (Dentin conditioner, GC Corp, Tokyo, Japan), are provided in Table 1.
Stimulated saliva was collected from 3 healthy, non-smoking adults who were over 20 years old and without any systemic disease. After explaining the details of the experiment and receiving their informed consent, their saliva was collected.
The specimens were randomly divided into 6 groups, with 10 specimens in each group. The experimental procedures are summarized in Fig. 1. Saliva contamination was performed with micro-brush for 20 sec. Water-rinsing, air-drying and polyacrylic acid (PAA) conditioning was performed for 10 seconds each. Group I was a control group where the specimens were conditioned with PAA. Groups II and III were contaminated with saliva before PAA conditioning. After saliva contamination, Group II specimens were air-dried, Group III specimens were rinsed with water and air-dried. Specimens in Groups IV, V, and VI were contaminated with saliva after PAA conditioning. After saliva contamination, Group IV specimens were dried, Group V specimens were rinsed with water and dried, and Group VI specimens were rinsed with water, dried, and additionally conditioned with PAA. After surface treatment, the dentin specimens were filled with a RMGI Restorative (Fuji II LC, GC Corp.) using a Teflon mold (5.0 mm in diameter and 1.5 mm height), and then light cured for 20 sec using an LED light curing unit (Bluephase, Ivoclar Vivadent, Schaan, Liechtenstein).
2. Shear bond strength (SBS) test
After bonding, all samples were stored in distilled water at room temperature for 24 h and then tested in shear mode on a universal testing machine (Instron, Canton, Mass). The specimens were stressed in an occluso-gingival direction with a crosshead speed of 0.5 mm/min.
3. Failure mode evaluation
Failure modes were evaluated using a field emission scanning electron microscope (Inspect F, FEI, USA) and classified as adhesive failure, mixed failure, or cohesive failure.
4. Statistical analysis
Statistical analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was performed to assess the regularity of the data. After confirming the regularity of shear bond strength, a one-way ANOVA was used with the Tukey test for post hoc analysis to compare the bond strength between groups. The differences in fracture mode between the groups were analyzed using Chi-squared analysis.
Ⅲ. Results
1. Shear bond strength
The mean bond strength of each group was as follows: 18.72 ±1.18 MPa in Group I, 13.09 ±1.36 MPa in Group II, 12.68 ± 1.20 MPa in Group III, 10.35 ± 1.32 MPa in Group IV, 11.17 ± 1.52 MPa in Group V, and 15.69 ± 1.31 MPa in Group VI (Table 2).
The shear bond strength varied significantly according to the decontamination method. Group I, which was the control group without saliva contamination, showed a significantly higher bond strength than the saliva contaminated groups (p= 0.001). Among the saliva contaminated groups, Group VI, which was subjected to additional PAA conditioning, showed significantly higher bond strength than the other contaminated groups (p= 0.001). Groups II and III, which were contaminated with saliva before PAA conditioning, showed higher bond strength than Groups IV and V, which were contaminated with saliva after PAA conditioning. There was no significant difference between Group II (washed and dried after saliva contamination) and Group III (only dried after saliva contamination) (p= 0.986). There was also no significant difference between Groups IV and V (p= 0.771).
Ⅳ. Discussion
RMGI has better mechanical properties compared to conventional glass ionomer cements. RMGI is commonly used in pediatric patients due to its fluoride releasing properties[7]. According to the American Academy of Pediatric Dentistry guidelines, RMGI is recommended as a material for Class I and Class II restorations in children with a high risk of caries[2]. It is also recommended to use RMGI as an interim therapeutic restoration in uncooperative patients or in those who have special healthcare needs[2].
In pediatric patients and people with lack of cooperation ability, it is often difficult to completely isolate the tooth from saliva. This study investigated the bond strength of RMGI to dentin according to when the saliva contamination occurred in combination with different decontamination methods.
PAA was first introduced by Powis et al.[8] as a conditioner to enhance the bond strength of glass ionomer cement to the tooth surface. In previous studies, the shear bond strength of RMGI to dentin with PAA conditioning was found to be significantly higher than that without PAA conditioning[3,9]. The guidelines for Fuji II LC, the RMGI used in this study, explain that PAA conditioning should be performed for 10 sec before the application of RMGI. Therefore, in this study, all dentin specimens were subjected to PAA conditioning according to the manufacturer’s guidelines.
In this study, the saliva contaminated groups showed significantly lower shear bond strength than the control group (p= 0.001). These results are consistent with previous studies[4]. RMGI contains resin components that induce mechanical bonding with the tooth surface. However, when the tooth surface is contaminated with saliva, the glycoprotein present in the saliva penetrates into the tooth surface, thereby interfering with the penetration of the resin component of RMGI. In addition, the penetrated glycoprotein prevents the polymerization of monomers and reduces bond strength[10,11].
In this study, Groups IV and V, which were contaminated with saliva after PAA conditioning, showed lower SBS values than Groups II and III, where the teeth were contaminated with saliva before PAA conditioning. The reason for this may be that PAA removed the salivary proteins, and salivary proteins on the tooth surfaces could not be removed by standard water-rinsing[12,13]. However, with additional acid conditioning, the organic remnants can easily be removed from the tooth surface by acid denaturation[14,15].
Previous studies showed that etching af ter saliva contamination can increase the bond strength, and this is consistent with our findings[16-18]. Group VI, which underwent additional PAA conditioning after saliva contamination, showed the highest SBS value among the saliva contaminated groups. There may be 2 reasons for this. First, it can be assumed that the salivary protein was removed by PAA conditioning. The second reason may be that, in Group VI, unlike the other groups, the conditioning time was 20 sec. Previous studies showed that conditioning with higher concentrations of etchant results in increased bond strength of RMGI[19]. In contrast, there was a study that showed that long etching time eliminates the calcium ions from the tooth surface, and weakens the chemical bonding of glass ionomer cements[20]. However, unlike the above studies, the tooth surfaces were contaminated with saliva in this study. Salivary proteins may have interfered with acid conditioning on the tooth surfaces that were contaminated with saliva. Previous studies have shown that the pH of teeth etched after saliva contamination was higher than that of teeth without saliva contamination[21]. Therefore, the long conditioning time in Group VI would have allowed proper surface conditioning. For the same reason, Groups II and III would have lower shear bond strength than that of the control group.
There were no statistically significant differences in the mode of fracture in all groups (p= 0.729). All groups showed mixed failure or adhesive failure, but no cohesive failure was observed. Groups I and VI had more mixed failures but these differences were not statistically significant. In previous studies, most of the failures between RMGI and dentin were adhesive and mixed failures[22].
In this study, saliva contamination reduced the shear bond strength of RMGI and it was significantly lower than that of the control group (p= 0.001). Rinsing and drying alone could not restore the bond strength of RMGI.
Additional PAA conditioning after saliva contamination improved the bond strength of RMGI, but this method still resulted in a bond strength lower than that of the group without contamination. Therefore, additional PAA conditioning was not a completely effective way to restore bond strength. Further studies are needed to analyze the treatment of saliva contaminated tooth surfaces during RMGI restoration.
Since this study was performed in vitro, the results may be different from those obtained in clinical settings. Further research investigating the effect of saliva contamination and decontamination methods on long-term bond strength of RMGI with pH cycling and similar conditions to the oral environment is warranted.
Ⅴ. Conclusion
Regardless of when the saliva contamination occurred, it adversely affected the shear bond strength of RMGI to dentin. Washing and drying of the saliva contaminated dentin did not improve the shear bond strength of RMGI. Additional PAA conditioning improved the shear bond strength when saliva contamination occurred after PAA conditioning, but shear bond strength was still lower than that in restorations without saliva contamination.