Molar-Incisor Malformation 환자의 유치열 내 전반적인 치수석 관찰의 증례 보고
Generalized Pulp Stones of Primary Dentition in a Patient with Molar-Incisor Malformation : A Case Report
Article information
Abstract
Molar-incisor malformation(MIM)은 최근 발견된 새로운 형태의 root anomaly로 제1대구치 및 제2유구치의 짧거나 미약한 치근 발달과 협착된 치수강, 그리고 cemento-enamel junction 높이에 석회화물질의 존재를 특징으로 한다.
이 증례는 MIM 이환 환자에서 유치열 전반에 걸쳐 치수강 내를 거의 가득 채우고 있는 free pulp stone을 방사선학적으로 확인할 수 있었다. 발치된 환자의 하악 제1대구치와 상악 제2유구치를 대상으로 micro-computed tomography(micro-CT) 촬영 및 scanning electron microscope-energy dispersive X-ray spectrometer 분석을 시행하였다. Micro-CT 영상을 통해, 하악 제1대구치의 치관 외부로 연결된 부근관의 존재가 확인되었다. 이것은 MIM 이환 치아에서 근관치료의 불량한 예후의 원인이 될 수 있을 것으로 사료된다.
Trans Abstract
Molar-incisor malformation (MIM) is a new type of root anomaly reported recently. The characteristics of MIM are dysplastic root formations, constriction of pulp chambers and presence of calcified matrices at the level of cementoenamel junction in permanent first molars and primary second molars. In some cases, permanent maxillary incisors are also affected.
The permanent first molars of the patient in this case report were affected with MIM. Generalized pulp stones were observed in overall primary dentition. Micro-computed tomography (micro-CT) imaging and scanning electron microscope-energy dispersive X-ray spectrometer analysis were performed on the extracted mandibular first molar and maxillary primary second molar of the patient. Micro-CT images revealed the discontinuity of enamel directly connected to an accessory canal of the root.
Ⅰ. Introduction
A new type of root anomaly has been reported in 2014 by Witt et al .[1]. Clinical and radiographic characteristics including dysplastic root formations, constrictions of pulp chambers and presence of calcified matrices at the level of cemento-enamel junctions (CEJ) in permanent first molars were observed[1]. In other studies, cervical constrictions of the crown and curved or shortened roots of permanent maxillary incisors have been reported in 40 - 58.3% of the patients[2-4]. In rare cases, affected permanent canines and mandibular incisors have also been reported[3,4]. By Witt et al .[1], the calcified matrix observed inside the pulp chamber was named cervical mineralized diaphragm (CMD). Lee et al .[2] named this anomaly as molar-incisor malformation (MIM), focused on the fact that clinical manifestations were confined to permanent first molars and permanent maxillary incisors. Wright et al .[3] suggested the term of molar root-incisor malformation (MRIM) pointing out that the crown portions of affected permanent first molars were intact. Thereafter, cases with similar clinical findings have been reported continuously[5-11].
In this case, generalized pulp stones of overall primary dentition were observed in the patient with MIM. Micro-computed tomography (micro-CT) images of extracted mandibular first molar revealed the presence of accessory canal directly opening into the outside of the crown.
Ⅱ. Case Report
A 5-year-old girl was referred to the Department of Pediatric Dentistry at Dankook University Dental Hospital with the chief complaint of thin and short roots of permanent first molars. Clinically, the crowns of permanent first molars were normal in shape. The patient had never experienced any clinical symptoms.
From the panoramic radiograph, ectopic eruption of mandibular left first molar and peg lateralis of maxillary right lateral incisor were observed (Fig. 1A). The roots of permanent first molars were thin and short (Fig. 1A). These clinical features coincided with the typical root morphology of teeth with MIM. Pulp chambers of mandibular primary second molars were constricted while other primary teeth showed normal morphology of pulp chambers (Fig. 1C, 1D). Generalized pulp stones were observed in the overall primary dentition and maxillary central incisors of the patient (Fig. 1A-1E). Past medical histories of the patient were reviewed. The patient was born at 39 weeks weighing 2.6 kg, which is within normal limits. At the first week after birth, the patient was diagnosed as intracranial hemorrhage. The patient had been hospitalized in neonatal intensive care unit (NICU) for 5 weeks.
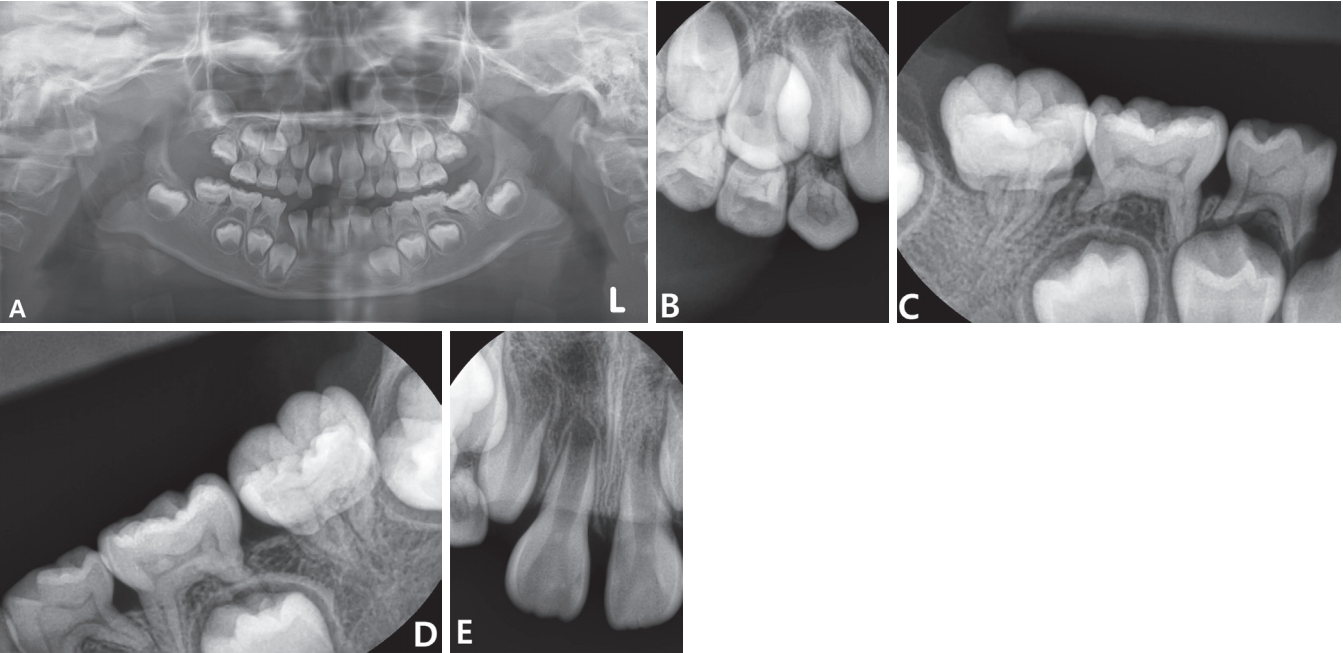
Radiographic features of the patient. (A) Initial panoramic view at the age of 5 years and 11 months. Permanent first molars show dysplastic root formations. Generalized pulp stones are seen in the pulp chambers of overall primary dentition. (B - E) Periapical view at the age of 6 years and 6 months. Pulp stones are observed in the pulp chambers of overall primary dentition and maxillary central incisors (B - E). The pulp chambers of mandibular primary second molars are constricted (C, D). Dysplastic roots of mandibular first molars are observed (C, D).
The patient was recalled every 3 - 6 months for routine dental check-ups. At the age of 8, the patient complained of severe pain in the right posterior teeth. Clinical and radiographic examinations were conducted to find the cause of the pain. Dental caries was not detected, but sensitivity to percussion and unphysiologic mobility of maxillary right first molar were observed. Maxillary right first molar was diagnosed as periapical periodontitis and has been extracted. For the next 11 months, the patient occasionally experienced dental pain on other permanent first molars. Consequently, remaining permanent first molars were extracted, with comprehensive orthodontic treatment plans also concerned.
With informed consent, micro-CT imaging and scanning electron microscope (SEM, SIGMA 500, Carl Zeiss, Jena, Germany)-energy dispersive X-ray spectrometer (EDX, NORAN System 7, Thermo Scientific, Madison, WI, USA) were conducted on the extracted maxillary right primary second molar and mandibular left first molar of the patient.
Micro-CT images revealed the presence of calcified matrix at the cervical 1/3 of the crown in mandibular left first molar. The radiopacity of the calcified matrix was observed to be between that of enamel and dentin (Fig. 2). Pulp chamber was irregularly constricted and the root canals were narrowed or partially obliterated (Fig. 2A). Unlike the previous studies that reported normal crown forms, the crown was constricted at the cervical 1/3 and prominence of enamel was observed underneath the constricted portion (Fig. 2A). At the constricted portion, the continuity of enamel was disrupted. This opening was directly connected to the accessory canal of the root.
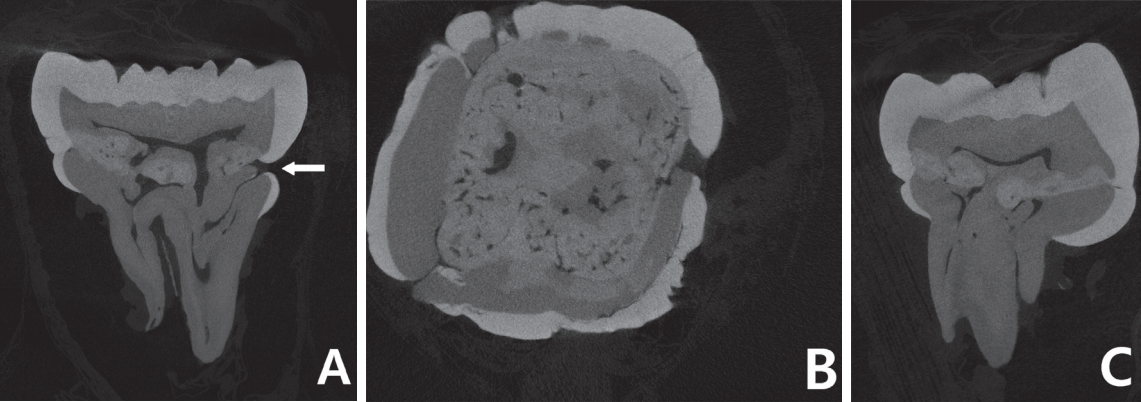
Micro-computed tomography (micro-CT) images of mandibular left first molar. (A) Sagittal section image. White arrow shows the discontinuity of the enamel and accessory canal is directly connected to the opening. (B) Transverse section image. (C) Coronal section image
In maxillary right primary second molar, multiple pulp stones were observed to fill in the pulp chamber (Fig. 3). The radiopacity of the pulp stones were similar to that of dentin (Fig. 3). Morphology of roots and canals could not be observed due to physiologic root resorption.
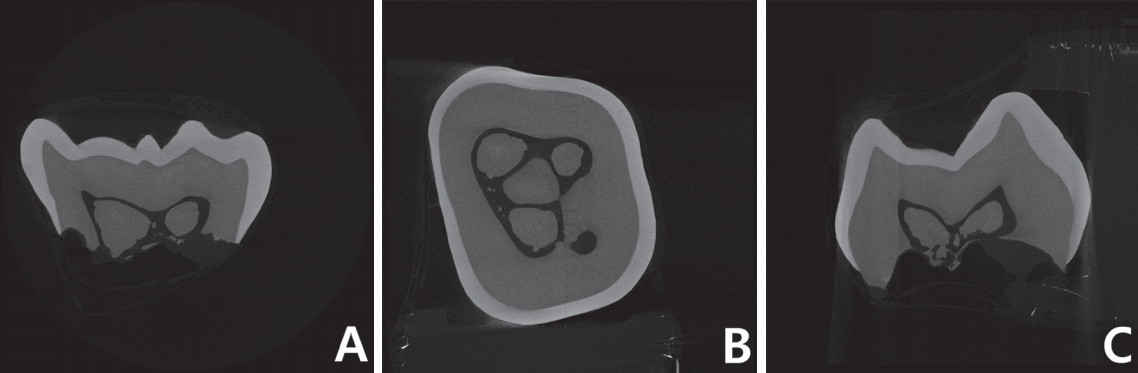
Micro-computed tomography (micro-CT) images of maxillary right primary second molar. Multiple free pulp stones are observed inside the pulp chamber. (A) Sagittal section image. (B) Transverse section image. (C) Coronal section image.
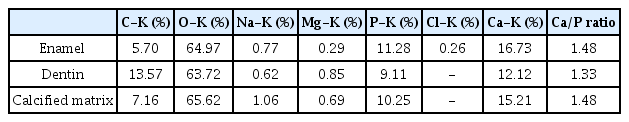
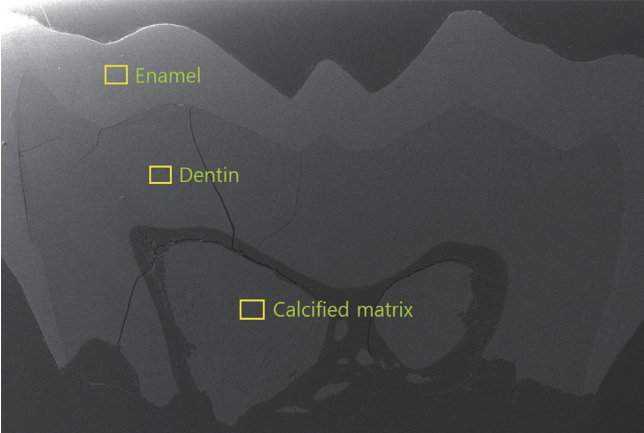
SEM-EDX analysis was performed to assess elemental compositions of enamel, dentin and calcified matrix inside the pulp chamber of mandibular left first molar (Fig. 4, Table 1) and maxillary right primary second molar (Fig. 5, Table 2).
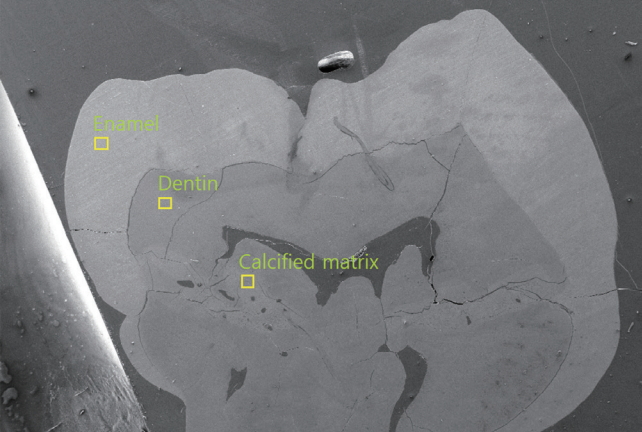
Scanning electron microscope (SEM) image of mandibular left first molar. Yellow boxes indicate the measurement points of each component. A layer of calcified matrix is observed across the pulp chamber at cervical 1/3 of the crown.
Elemental composition of mandibular first molar by energy dispersive X-ray spectrometer (EDX) analysis. The results are based on atom%
Scanning electron microscope (SEM) image of maxillary right primary second molar. Yellow boxes indicate the measurement points of each component. Multiple free pulp stones are observed inside the pulp chamber.

Elemental composition of maxillary right primary second molar by energy dispersive X-ray spectrometer (EDX) analysis. The results are based on atom%
The elemental composition of the calcified matrix in the pulp chamber of mandibular left first molar was different from that of dentin in the same tooth. Ca/P ratio of the calcified matrix was identified to be more similar to that of enamel, rather than dentin (Table 1). In maxillary right primary second molar, the Ca/P ratio of the calcified matrix in the pulp chamber was assessed to be slightly lower than that of dentin in the same tooth (Table 2).
Ⅲ. Discussion
The etiology of MIM remains unclear. But medical conditions during the first 2 years after birth rather than genetic factors are considered to be the causative factors to this anomaly[1-3,5,9]. Recently, a case of a patient with MIM whose monozygotic twin had normal dental development has been reported[9]. Most patients with MIM were reported to have experienced major medical conditions during the first 2 years after birth[2,3]. Although central nervous system (CNS) disorders including meningomyelocele, meningitis, spina bifida, cerebral cyst are most frequently reported, various other conditions such as premature birth and renal disease have been also reported in these patients[2,3]. In this regard, there is a hypothesis that MIM may be related to the high dose of antibiotics, rather than medical conditions themselves[4]. Also, clinical features of MIM are expressed in only few patients with same medical conditions. This suggests that certain genetic predispositions combined with environmental stressors may be the cause of MIM[3].
There is a time gap between the patients’ past medical histories and developmental period of mainly affected teeth. On this issue, Wright et al .[3] insisted that environmental stressors may influence specific cell line and these effects grow over time, resulting in abnormal development of the roots. Brusevold et al . [6] suggested that Hertwig’s epithelial root sheath (HERS) may be affected by the stressors long before the root development and later be developed, resulting in impaired differentiation of odontoblasts.
EDX analysis is an effective tool for investigating the elemental compositions of hard tissues. The Ca/P ratio from the result represents the level of mineralization or calcification[12,13]. Ca/P ratio of calcified matrix in mandibular left first molar was assessed to be similar to that of enamel. It corresponds to the previous study in which the Ca/P ratio of “brighter tissue” in CMD was similar to that of enamel[8].
In this case, generalized pulp stones of overall primary dentition and maxillary central incisors were observed on radiographic examination. The pulp chambers of both anterior and posterior teeth were filled with pulp stones. The pulp stones were separated from the dentinal walls. Analysis of the pulp stones inside the pulp chamber of maxillary right primary second molar was also performed. Radiopacity of the pulp stone was similar to that of dentin. On EDX analysis, Ca/P ratio of the pulp stone was slightly lower than that of dentin.
Histologically, pulp stones can be classified into ‘true pulp stones’ and ‘false pulp stones’. Other types called ‘diffuse’ or ‘amorphous’ are more irregularly formed and commonly found around the blood vessels[14,15]. On the basis of locations, pulp stones can be divided into ‘free’ pulp stones and ‘adherent’ or ‘embedded’ pulp stones[14,15]. The majority of pulp stones found during radiographic examinations are free pulp stones[14,15]. True, free pulp stones are reported to be more frequent in teeth where root development is incomplete[15]. Prevalence of pulp stones in primary teeth is lower than in permanent teeth, and is reported to be 5.8 - 6.7%[16,17].
A case presenting pulp stones in permanent maxillary incisors with MIM has been reported[10]. However, the pulp stones in the previous case report were confined to permanent maxillary incisors. In this case, generalized pulp stones were also observed in overall primary dentition of a patient with MIM. Etiology of pulp stones is still unclear. According to previous studies, the prevalence of pulp stones tends to increase with age, and is related to local factors such as trauma, periodontal disease, low-intensity stimuli[18]. But cases with generalized pulp stones are rare, and reported to be commonly related to systemic conditions such as tumoral calcinosis, dentin dysplasia type II, dentinogenesis imperfecta, cardiovascular diseases[15,18]. Rarer cases with idiopathic type have been also reported, which is thought to be associated with genetic predisposition[15]. The patient in this case report had medical history of intracranial hemorrhage after birth. But correlation between this condition and formation of generalized pulp stones has not been established.
MIM is reported to affect specific types of teeth. The developmental periods for these teeth are relatively similar. In primary second molars, formation of the furcation starts at around 1.3 years after birth and the root formation continues until 3 years of age[19]. In maxillary permanent incisors, calcification of crown is initiated at around 3 - 4 months after birth and is finished at around 4 - 5 years of age[20]. The formation of roots in permanent first molars starts at around 2.5 - 3 years after birth[20]. At this period, most teeth in primary dentition would have accomplished the crown and root formation[19]. The same stressors associated with MIM may have contributed to the formation of generalized pulp stones in primary dentition in this case. However, it is hard to draw a definite conclusion from a single case.
In most previous studies, permanent first molars with MIM were reported to be normal in crown morphology[1-7]. However, in some cases, abnormalities such as prominence or hypomineralization of enamel have been reported[6,8], which is similar to the findings in this case. On micro-CT images, the crown of mandibular left first molar was constricted at the cervical 1/3 and prominence of enamel was observed underneath. In addition, discontinuity of enamel was observed at cervical 1/3 of mesial surface, and an accessory canal of mesio-lingual root was directly connected to this opening (Fig. 2A).
Due to its irregularly constricted pulp chambers and thin, shortened roots, teeth with MIM have poor prognosis of endodontic treatment. In many cases, periapical abscesses combined with severe alveolar bone resorption in teeth without dental caries have been reported[4,7-9]. In few cases, successful endodontic treatments in patients with MIM have been reported[4,11]. However, in most cases, permanent first molars with MIM have been extracted [7,8]. The discontinuity of enamel found in this study may explain the reason for the formation of periapical abscess and poor prognosis of endodontic treatment in teeth with MIM. In this regard, clinicians should be aware of high morbidity of teeth with MIM, and timely intervene for development of sound occlusion in these patients.
Ⅳ. Summary
In this case, generalized pulp stones in overall primary dentition were observed. It suggests that the same stressors which lead to expression of MIM in permanent teeth may also have affected primary teeth whose root and crown formation had already been complete. Micro-CT images on mandibular first molar revealed the presence of discontinuity of enamel in crown portion which accessory canal was directly connected to. This finding may provide an explanation for the development of periapical abscess without dental caries and poor prognosis of endodontic treatments in teeth with MIM.
