포도당-6-인산탈수소효소 결핍증 환아의 치과적 관리
Dental Management in a Child Patient with Glucose-6-phosphate Dehydrogenase Deficiency : A Case Report
Article information
Abstract
포도당-6-인산탈수소효소 결핍증은 X 염색체 열성으로 유전되는 세계에서 가장 흔한 효소 결핍증이다. 이 질환은 우리나라에서는 드물게 나타나지만, 다문화 가정의 증가로 유병률이 높아질 가능성이 있다. 이 결핍증의 주된 문제는 소아치과에서 일반적으로 사용 되거나 처방되는 일부 약물에 의해 용혈성 빈혈이 유발될 수 있다는 점이다. 소아치과 의사는 환자의 병력에 대한 정확한 지식을 갖고 용혈을 유발할 수 있는 산화 스트레스를 피하기 위해 소아 혈액 전문의와 상담해야 한다. 가장 효과적인 치료는 빈혈을 유발할 수 있 는 인자에 노출되지 않도록 예방하는 것이다. 이 질환으로 진단받은 환자들에게 위험인자에 대한 적절한 교육은 필수적이다. 이 증례 보고는 G6PD 결핍증 환아에서 주의해야 할 약물과 치과적 관리 방법에 대해 논의하고자 하였다.
Trans Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an X-linked recessive disorder and is the most common enzyme deficiency worldwide. Although this disease is rare in Korea, its prevalence may increase due to an increase of multicultural families. Patients with this deficiency are prone to hemolytic anemia provoked by specific drugs commonly used or prescribed in pediatric dentistry. It is necessary for pediatric dentists to have accurate knowledge of a patient's medical history and to consult with a pediatric hematologist to avoid oxidative stress that can lead to hemolysis. The most effective treatment is prevention of exposure to factors that may trigger anemia. Appropriate education regarding risk factors is essential for patients diagnosed with this disease. This case report aimed to discuss the drugs and dental management methods that should be cautious in children with G6PD deficiency.
Ⅰ. Introduction
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme that catalyzes the first reaction in the pentose phosphate pathway. G6PD converts glucose-6-phosphate into 6-phosphogluconolactone, which converts glucose to pentose sugars for nucleic acid synthesis[1]. This step leads to the concomitant reduction of nicotinamide adenine dinucleotide phosphate (NADP) to its reduced form (NADPH), which helps preserve the reduced form of glutathione (GSH)[2,3]. GSH functions as an antioxidant and eliminates hydrogen peroxide (H2O2) from cells to protect against oxidative damage[3]. Therefore, deficiency of G6PD may result in the excessive accumulation of H2O2 and oxidative damage, which can lead to an abnormal destruction of cell walls (Fig. 1). In most cells, this deficiency is inconsequential since other mitochondrial processes sustain a supply of natural antioxidants to eliminate damaging reactive oxidative species[4]. However, as red blood cells (RBCs) do not contain mitochondria, the pentose phosphate pathway is their only source of NADPH[1,5]. Therefore, defense against oxidative damage is strongly dependent on G6PD activity in RBCs.
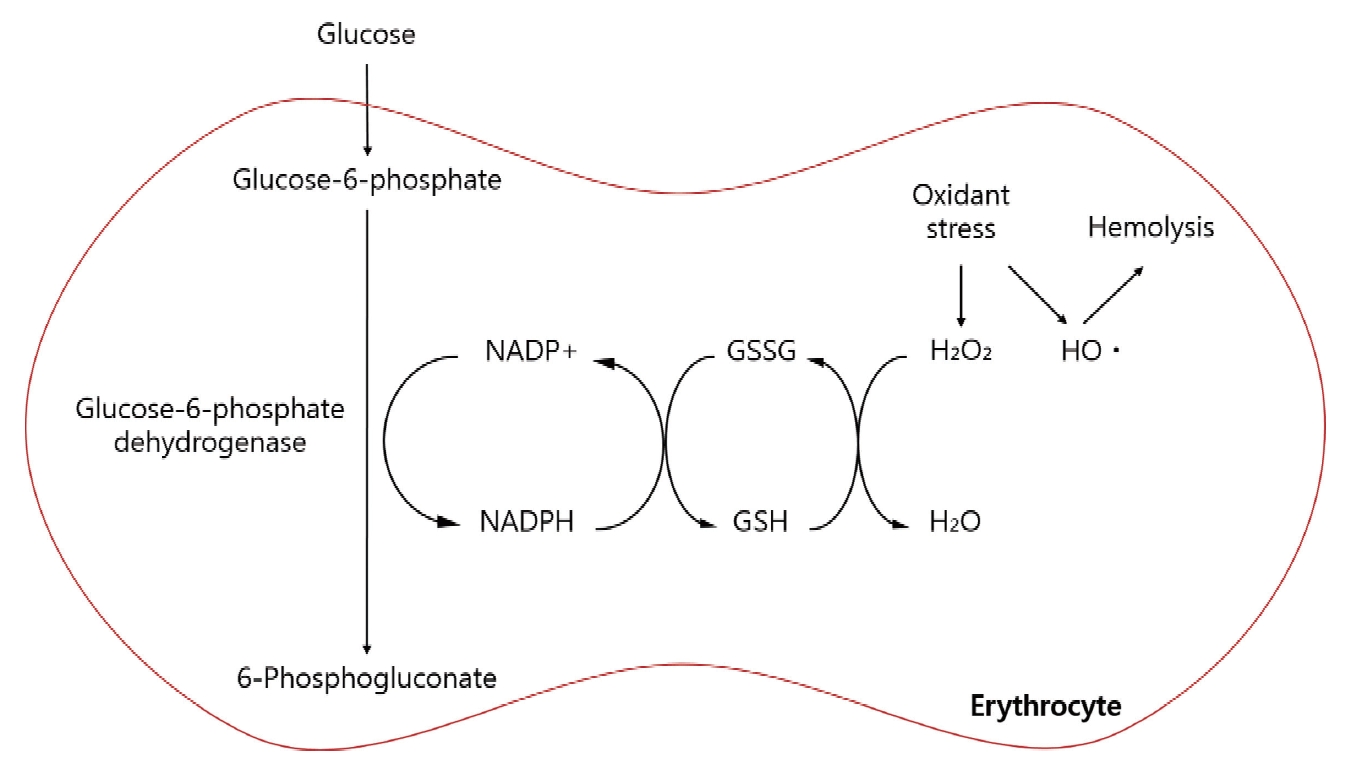
Pentose phosphate pathway in red blood cells.
NADP+ = Nicotinamide adenine dinucleotide phosphate, NADPH = Reduced nicotinamide adenine dinucleotide phosphate, GSSG = Oxidized glutathione, GSH = Reduced glutathione
G6PD deficiency is the most common human enzyme defect, and more than 400 million people worldwide have this deficiency. The prevalence of G6PD deficiency differs according to geographical location, with the highest prevalence reported in Africa, southeast Asia, and the Mediterranean region[1].
Since the first case was reported in 1990[6], a few reports have been published in Korea, although none have been published in the field of pediatric dentistry. This report presents a rare case of a child with G6PD deficiency and discuss the main considerations and precautions to take into account during dental treatment for pediatric patients.
Ⅱ. Case Report
A 4-year-old boy visited the Department of Pediatric Dentistry at Chonnam National University Dental Hospital with a chief complaint of multiple caries. The patient had a history of hemolytic anemia of unknown cause, that persisted despite iron supplementation. After several hematologic tests, the child was diagnosed with G6PD deficiency at the age of 2 year and 9 months. No medication for G6PD deficiency was prescribed to the patient and the patient was advised not to take any drugs or consume food that could cause hemolysis.
On intraoral examination, multiple dental caries, poor oral hygiene with moderate plaque accumulation, and right posterior crossbite were detected (Fig. 2). The parents mentioned that the patient experienced mild dental pain and bleeding during toothbrushing. On radiographic examination, multiple dental caries and root resorption of the maxillary primary central incisor due to severe caries were observed (Fig. 3).

Initial intraoral photographs prior to treatment. (A) Crossbite on the right posterior teeth. (B, C) Multiple dental caries were observed.
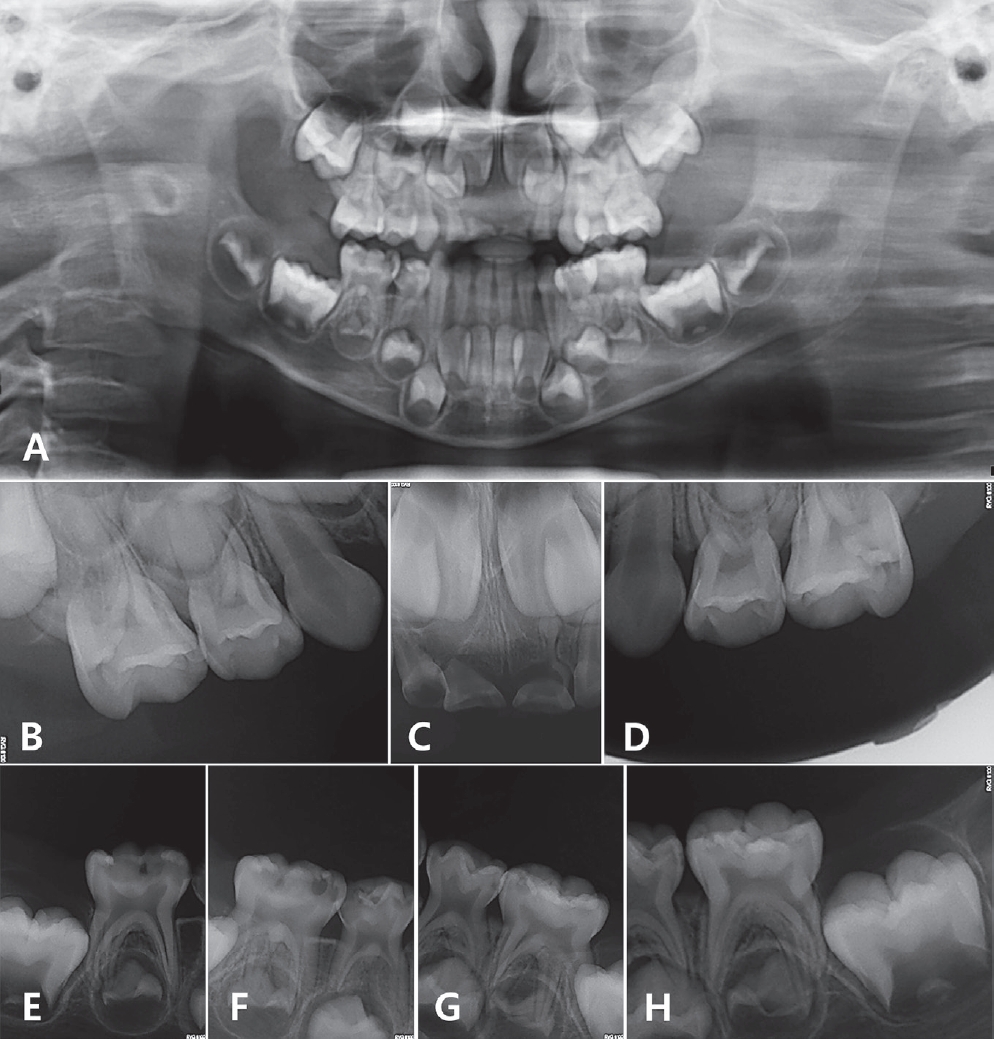
(A) Initial panoramic radiograph of the patient. (C) Root resorption of the maxillary primary central incisor. (B, D-H) Dental caries on all primary molars.
The patient’s compliance was evaluated as 2nd grade by Frankl behavioral classification. The patient had never been treated under anesthesia or sedation. Given the patient’s lack of cooperation, sedative treatment was planned. The patient’s systemic condition and therapeutic approaches, such as precautions related to using sedative medication, were discussed with a pediatric hematologist. Avoiding the use of midazolam as a sedative agent and lidocaine as a local anesthetic agent was recommended. Based on the information collected, treatment using chloral hydrate, hydroxyzine, and nitrous oxide (N2O) inhalation sedation was decided. The patient’s parents fully understood the procedure and agreed to the planned treatment by signing a written informed consent. The patient was orally administered 1.5 g chloral hydrate (Pocral®, Hanlim, Korea) and 20 mg hydroxyzine (Dudrizine®, Sama, Korea), and the N2O concentration was maintained at 40% during treatment. The treatment involved extractions, pulp therapy, and restorations was performed under local anesthesia using a total 3.6 mL of bupivacaine. The procedure proceeded smoothly without any problems. In order to monitor any hemolytic attacks, the patient and his parents were advised to visit a pediatrician 3 days after the dental treatment.
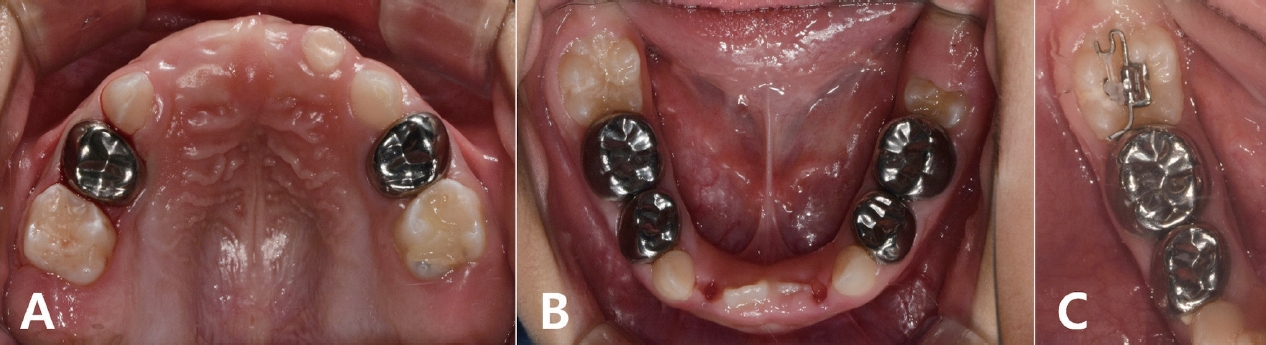
Periodic follow-up was performed every 3 months (Fig. 4A, 4B). During the regular examination, an ectopic eruption of the mandibular right first molar was observed, and a piston elastic appliance was used for molar uprighting (Fig. 4C). Orthodontic treatment for crossbite of the right posterior teeth is planned to initiate around the time the patient turned 7 years old.
Ⅲ. Discussion
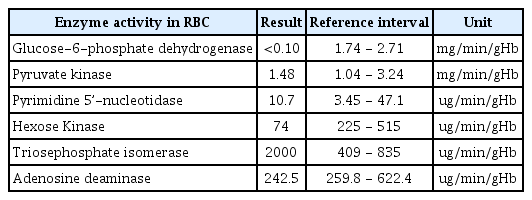
G6PD deficiency is an X-linked, hereditary genetic defect that has a higher incidence in males than in females[1]. A son can acquire this disease if his mother is an asymptomatic carrier. In the current case, the patient was born between a Korean father and a Filipino mother, and it was presumed that he inherited a genetic factor from his mother. The patient's mother also had a history of anemia. Diagnosis of the patient was confirmed by using ultra performance liquid chromatography-tandem mass spectrometry, which is a method used to measure enzymatic activity in RBCs. The patient’s test result revealed a decrease in G6PD activity (Table 1).
Blackwell et al .[7] reported that the incidence of G6PD deficiency was estimated to be less than 0.9% in Korean males. Saha[8] reported a high frequency of G6PD deficiency in Singaporean Chinese, Thai, Filipino, and Taiwanese individuals, ranging from 6.7 - 13.0%, while Koreans had a low frequency of 3.5%. The recent studies failed to detect any known G6PD deficiency variants in the Korean population, due to the small study population[9,10]. It is difficult to determine the exact prevalence of G6PD deficiency in Korea based on the reported content so far.
According to a recent report from the Ministry of Justice's Yearbook of Immigration and Foreigners Policy[11], the number of foreigners and immigrants residing in Korea has been increasing every year. The proportion of multicultural births was highest in Jeonbuk at 8.1%, followed by Jeju and Jeonnam at 7.8%[12]. Nationality of foreigners and naturalized immigrants who gave multicultural birth is in the order of Vietnam, China, Philippines[12]. These countries have a high prevalence of G6PD deficiency, with Vietnam 0.4 - 9.1%, China 1.6 - 9.0%, and the Philippines 4.5 - 25.7%, respectively[13,14]. This can leads to ethnic diversity and an increase in the number of multicultural families. So the prevalence of G6PD deficiency in Korea may increase in the near future.
This disorder exhibits no over t or distinctive clinical signs[15]. It is difficult to detect if the patient has not yet been diagnosed with G6PD deficiency. Newborn babies with severe neonatal jaundice, particularly those of Mediterranean or African ancestry, are quite likely to have G6PD deficiency[1]. Data from a series of studies suggest that about a third of all male newborn babies with neonatal jaundice have G6PD deficiency[16-18]. At the time of history taking, it is necessary to differentially diagnose G6PD deficiency in patients with neonatal jaundice or symptoms of anemia without a clear cause. Therefore, thorough and careful medical and family history taking are particularly important.
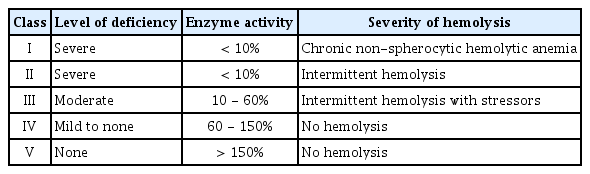
The severity of the disease is determined by the magnitude of enzyme deficiency. As for the genotype-phenotype correlations, the World Health Organization has categorized G6PD deficiency into 5 classes (Table 2) based on residual enzymatic activity and clinical manifestations[19]. In this case, the patient was categorized with a class II disorder.
Fortunately, most G6PD-deficient patients usually have no clinical manifestations and remain asymptomatic. The disorder generally manifests as acute hemolysis, which arises when RBCs undergo oxidative stress triggered by agents such as drugs, infection, or the ingestion of fava beans[1]. There is considerable debate among researchers as to which drugs should be avoided (Table 3) in patients with G6PD deficiency[20]. Previous reports have recommended avoiding drugs that cause oxidative stress and induce methemoglobinemia[21,22]. Local anesthetic agents, such as prilocaine, articaine, and lidocaine, and topical anesthetics, such as benzocaine, have been reported to induce methemoglobinemia in patients with G6PD deficiency[23,24]. Altikat et al .[25] studied the effects of certain anesthetic agents on the enzymatic activity of G6PD in vitro. It was found that isoflurane, sevoflurane, diazepam and midazolam had an inhibitory effects on G6PD activity, whereas halothane and ketamine had no effect on G6PD activity. In this case, based on previous reports and advice from a hematologist, sedation was performed using chloral hydrate, hydroxyzine, and N2O instead of midazolam, and bupivacaine was selected as a local anesthetic instead of lidocaine.
Sklar[26] reported that acetaminophen overdose as a cause of hemolysis in patients with G6PD deficiency. There are several reports suggesting that acetaminophen can be administered safely if recommended doses are not exceeded[22,27]. Najafi et al .[28] conducted a study to evaluate the risk of hemolysis after short-term administration of analgesics, including paracetamol, ibuprofen, tramadol, sufentanil and parecoxib, in therapeutic dosages in children with G6PD deficiency. They reported that short-term administration of these drugs in therapeutic dosages did not increase the risk of hemolysis in the study population.
Whether a specific drug directly causes a hemolytic crisis in patients with G6PD deficiency is often difficult to establish. As pharmacokinetics can vary among individuals, an agent that appears to be safe in some individuals may not necessarily be safe for all. It is important to understand that a few case reports describing drug-disease interactions are not sufficient to establish causality[4].
According to Elyassi and Rowshan[22], infection is probably the most common factor that induces hemolytic anemia in patients with G6PD deficiency. While the mechanism underlying this reaction is not yet known, it is theorized that during phagocytosis, leukocytes release active oxygen species that cause oxidative stress in RBCs[1-3]. Quereshy et al .[29] reported cases of patients with G6PD deficiency who developed hemolytic anemia secondary to maxillofacial infection caused by severely decayed teeth. In order to prevent infection, the patient in this case and his parents were given instructions on how to brush effectively to maintain oral hygiene. It was monitored through regular check-ups, and these instructions were reiterated at every visit. Additionally, the patient was recommended to undergo fluoride application every 3 months after the initial treatment.
Clinical signs and symptoms of hemolysis typically occur within 24 - 72 hours after exposure to a triggering agent[22]. This makes it difficult for a dentist to identify a hemolytic crisis in patients who visit the outpatient clinic for a short time. General anesthesia typically masks the immediate signs of hemolysis[30]. Dentists should inform high-risk patients and their parents to be aware of signs and symptoms of a hemolytic crisis[22]. The clinical manifestations of hemolytic anemia include a sudden increase in temperature, nausea, vomiting, cyanosis, headache, fatigue, dyspnea, and dark urine[30,31]. It is also important to maintain a close follow-up to check for the occurrence of hemolytic crisis. In this case, there were no physical symptoms indicative of hemolytic anemia during the 1 week follow-up.
In most cases of acute hemolysis, no specific treatment is necessary[22]. Treatment of hemolysis consists of eliminating the triggering factor and controlling the clinical symptoms[3]. Hemoglobin levels recover after 8 - 10 days[1,22]. The most effective management strategy for G6PD deficiency is to prevent hemolysis by avoiding oxidative stressors[1,3]. Clinicians and patients must stay alert and prepare to avoid any factors that may trigger severe clinical manifestations of the deficiency.
Ⅳ. Summary
Although G6PD deficiency is a very rare disease in Korea, its prevalence might increase due to the immigrant from different ethnic origins and geographical regions and the consequent increase of multicultural families. Pediatric dentists should be able to recognize children with suspected G6PD deficiency through thorough medical history and family history taking. In cases of treatments that may lead to pain and anxiety in children, since the procedure itself can induce oxidative stress, stress relieving methods for patients should be considered. Pediatric dentists should consider that drugs commonly used in dental treatment can cause hemolysis in patients with G6PD deficiency, and knowledge of this disease is essential for planning and safe management of pediatric patients.