하악 결합 부위에 발생한 외상성 골낭 : 증례보고
Traumatic Bone Cyst in the Mandibular Symphysis : Case Reports
Article information
Abstract
외상성 골낭은 임상 증상이 없으며 청소년기에 호발 한다. 주로 정기검진을 위한 파노라마 촬영시에 우연히 발견되며 병소는 비교적 경계가 뚜렷한 단일성의 방사선 투과상으로 가리비 껍질 모양의 변연을 보인다. 조직학적 소견으로 해면골내 공동과 얇고 느슨한 결합조직의 이장이 관찰된다. 상피세포의 이장이 존재하지 않는 것이 주된 조직학적 특징이다. 호발 부위는 하악 견치부와 제3대구치 사이이다. 하악 결합 부위에서는 드물게 나타난다. 외상성 골낭의 발병원인은 아직 명확하지 않다. 외상성 골낭은 외과적 적출술과 소파술로 치료되며 외과적인 처치 후 6 - 12 개월 정도 지나면 새로운 골로 채워진다. 대부분의 케이스에서 치료 전 외상성 골낭으로 진단 내리는 것은 한계가 있다. 따라서 본 증례에서는 방사선학적, 조직학적 검사를 통해 진단 내렸다. 두 환자 모두 외상 관련 병력은 없었다. 외과적 처치 후 파노라마와 CBCT를 이용하여 골성 치유를 확인하였다.
Trans Abstract
Traumatic bone cyst (TBC) is an asymptomatic lesion seen most in adolescents. TBC is found incidentally on routine panoramic examinations and appears as a relatively well-demarcated unilocular radiolucency with scalloped margins. Histological examination reveals a vacant cavity of cancellous bone usually unlined or very occasionally lined with a thin connective tissue layer. The lack of lining epithelial membrane is common histological feature. The most affected site is between the mandibular canine and third molar. The involvement of the mandibular symphysis is rare. The etiopathogenesis of the TBC is unclear. TBC is treated with surgical exploration and curettage; new bone is formed in place of the lesion within 6 - 12 months of surgery. Diagnosis of TBC prior to surgical intervention has limitations in most of the cases. Both of our patients were diagnosed through radiological examination and biopsy. Neither patient had a history of trauma. After surgery, the panoramic radiograph and CBCT were used to confirm bone healing.
Ⅰ. Introduction
Traumatic bone cysts (TBCs) were first described by Lucas in 1929[1]. Since then, the detection rate of TBC has gradually increased due to advances in panoramic imaging techniques. Because the etiology of the lesion is uncertain, TBCs are also called traumatic cysts, simple bone cysts, solitary bone cysts, hemorrhagic bone cysts, hemorrhagic cysts, and idiopathic bone cavities[2].
TBCs account for 1% of all cysts found in the jaw[3]. Of the bone cysts found in the jaw, 89% are in the mandible and 11% in the maxilla. Mandibular cysts are most found in the region between the mandibular canine and third molar, and are rarely seen in mandibular symphysis[4].
TBCs are usually asymptomatic and often detected incidentally during routine radiographs. They are seen most frequently in people in their second decade of life[5-7]. Sex predilection is controversial. Some studies stated no gender predilection, other studies stated a male predilection[8,9].
The World Health Organization (WHO) classifies TBCs within a group of bone-related lesions along with aneurysmal bone cyst, ossifying fibroma, fibrous dysplasia, osseous dysplasia, central giant cell granuloma, and cherubism. TBC is a pseudocyst devoid of an epithelial lining, and consists of an intrabony cavity lined by a thin layer of connective tissue[10]. The typical radiographic appearance of a TBC is of a well-defined unilocular radiolucent lesion varying in size and, if it extends into the intraradicular space, having interdental scalloped margins[11,12].
The mainstay of treatment for TBCs is surgical exploration of the lesion followed by curettage of the bony cavity walls to cause hemorrhage[6,13,14]. In some cases, the lesions may heal without surgical intervention. However, surgery is recommended to prevent disease processes and promote healing[15]. The lesion recurrence rate is approximately 2%[16].
TBCs of the two patients were located in the mandibular symphysis. After surgical curettage, the platelet-rich fibrin (PRF) was used in the first patient, and the resorbable collagen matrix was used in the second patient.
Ⅱ. Case Reports
1. Case 1
An 8-year-old child presented to our clinic for dental caries. A panoramic radiograph taken to assess general oral health revealed a round radiolucent lesion in the region of the mandibular incisors (Fig. 1). The patient was advised to attend regular follow-up clinical visits so that changes in the size of the lesion could be monitored. Despite of 3 year follow-up, any spontaneous healing was not shown.
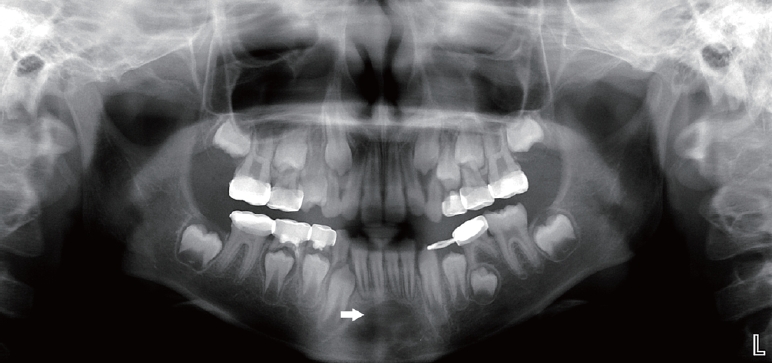
The first preoperative panoramic radiograph taken at age 8 showed a well-defined radiolucent lesion (white arrow) in the mandibular symphysis in case 1.
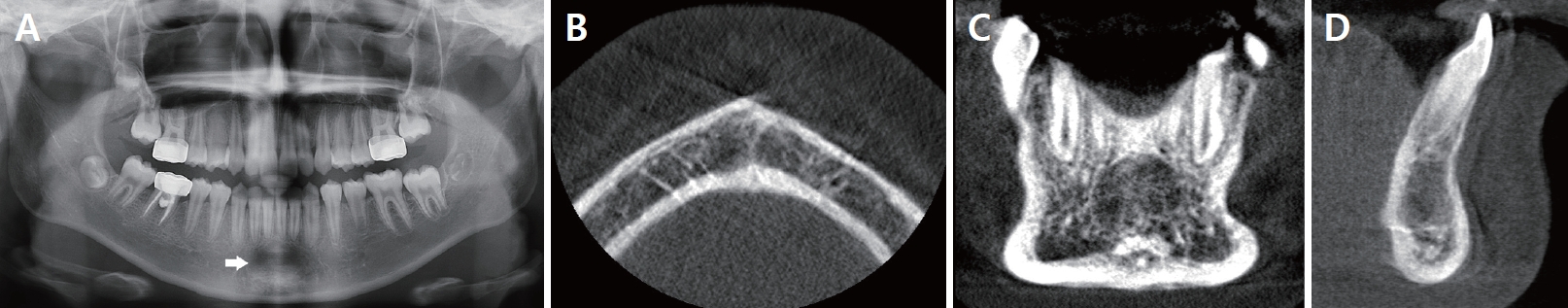
The radiolucent area increased in size on the follow-up panoramic image, and a cone beam computed tomography (CBCT) scan was performed to confirm the diagnosis. The CBCT image showed relatively well-demarcated radiolucency surrounded by a partial hyperostosis of the border in the anterior region of the mandible, from the right mandibular lateral incisor to the left mandibular lateral incisor (Fig. 2). The patient had no systemic disease or familial medical history. There were no symptoms such as pain, facial swelling, teeth mobility and root resorption. The adjacent teeth (#33, 32, 31, 41, 42, and 43) responded normally to electric pulp test (EPT) and non-tender on percussion. The patient was preliminary diagnosed with TBC and biopsy were planned to confirm the diagnosis. The patient and her guardian denied any history of trauma.

The second preoperative panoramic radiograph taken at age 11 (A) showed an increase in the size of the radiolucent lesion. The first preoperative CBCT image taken at age 11 showed (B-D) a relatively well-demarcated radiolucent lesion with a size of 10.77 × 10.70 × 8.89 mm surrounded by a partial hyperostosis of the border in the anterior region of the mandible in case 1.
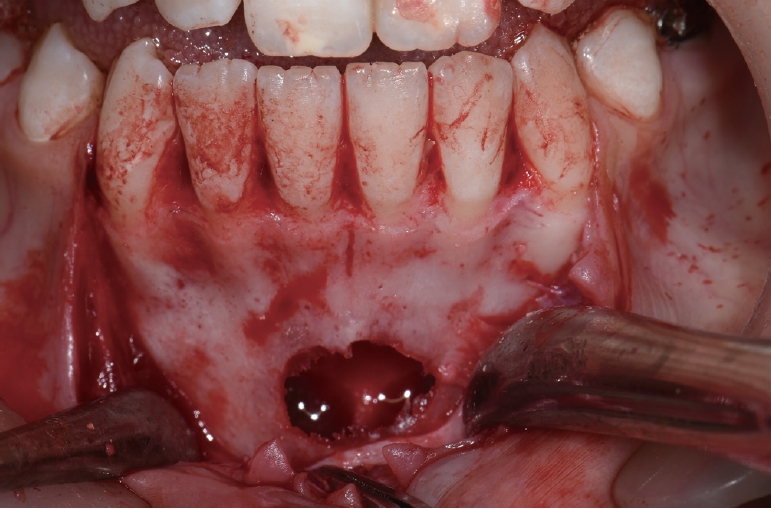
Platelet-rich fibrin (PRF) was prepared using a centrifugation device (Duo® Quattro, Avec surgical, Nice, France). Around 10mL of intravenous blood was drawn into tube without anticoagulant and centrifuged at 1300 revolutions per minute (rpm) for 8 minutes. Under local anesthesia, a sulcular incision was made from the right mandibular canine to left mandibular canine, and a vertical incision was made on the distal aspect of the two teeth to form a labial flap. After performing osteotomy using a round bone-cutting surgical bur, the lesion was curetted to cause hemorrhage and the tissue removed during curettage was sent to the laboratory for histological examination. The surgical site was rinsed with normal saline, and 10 cc of PRF was injected into the cavity (Fig. 3). The histologic examination revealed thin connective tissue lining the lumen, areas with thin fibrin layers, and hemosiderin deposits facing the lumen, suggestive of a TBC (Fig. 4). Postoperatively, the patient was monitored for wound healing. One year after surgery, a panoramic radiograph and CBCT scan showed an increase in the radiopacity within the lesion (Fig. 5).
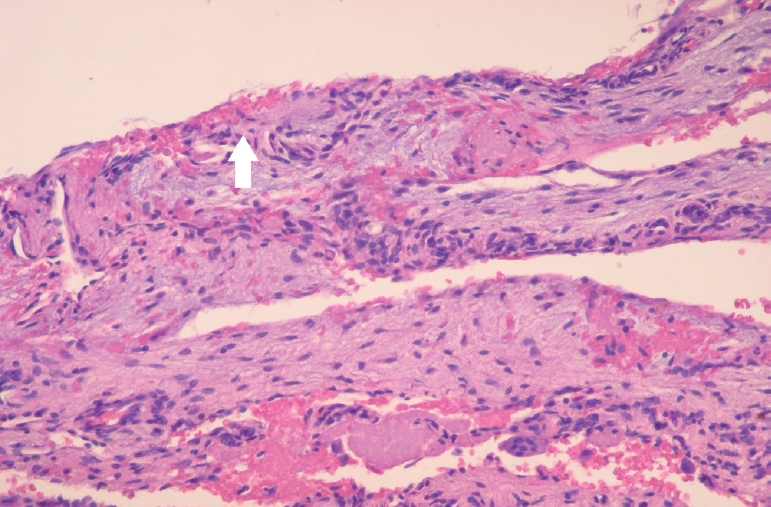
Histologic examination revealed thin connective tissue lining the lumen, and areas of thin fibrin layers with hemosiderin (white arrow) deposits facing the lumen.
2. Case 2
A 14-year-old girl was referred to our department by her dentist. The patient visited a local dental clinic for a routine check-up, and a panoramic radiograph revealed a diffuse radiolucent mandibular lesion. The CBCT scan performed at that time showed a well-demarcated radiolucent lesion in the anterior region of the mandible, from the right mandibular central incisor and the second premolar that extended to the inferior margin of the mandible. The upper margin of the lesion was corticated and extended into the roots of the teeth. The lesion appeared to bulge due to the thinned inferior margin of the mandibular cortical bone (Fig. 6). All adjacent teeth (#41 - 45) responded normally to EPT and non-tender to percussion. Lamina dura of all teeth involved were found to be intact. The patient denied any history of trauma. The patient had good oral hygiene with no systemic disease or familial medical history.
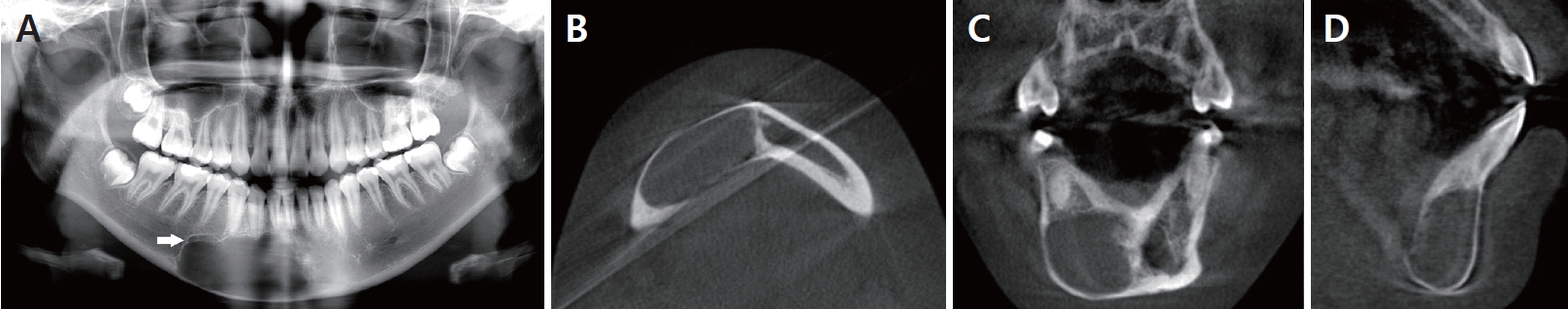
The preoperative panoramic radiograph taken at age 14 (A) showed a unilocular radiolucent lesion with well-defined scalloped borders in the mandibular anterior region extending from central incisor to distal aspect of second premolar. The preoperative CBCT image taken at age 14 (B-D) showed a well-demarcated unilocular radiolucent lesion with a size of 29.35 × 19.57 × 14.36 mm in the anterior region of the mandible with no perforation of labial or lingual cortical bones in case 2.
Our differential diagnoses included TBC and central giant-cell granuloma. Biopsy and surgery were planned to confirm the diagnosis and remove the lesion. Under local anesthesia, a sulcular incision was made from the right mandibular central incisor to the second premolar, and a vertical incision was made on the distal aspect of the two teeth to form a labial flap.
After osteotomy, the inner surface of the TBC was curetted using a surgical curette. The surgical site was rinsed with normal saline, and covered with the resorbable collagen matrix (Ossix Plus®, Datum Dental Biotech, Lod, Israel). It was used to barrier the surgical site and promote bone healing process. The tissue obtained from the curettage was sent for histological examination, which confirmed the diagnosis of TBC (Fig. 7). Six months later, a panoramic radiograph showed a decrease in radiolucency. One year later, a panoramic radiograph and CBCT scan showed an increase in radiopacity within the lesion, confirming healing of the bone (Fig. 8).
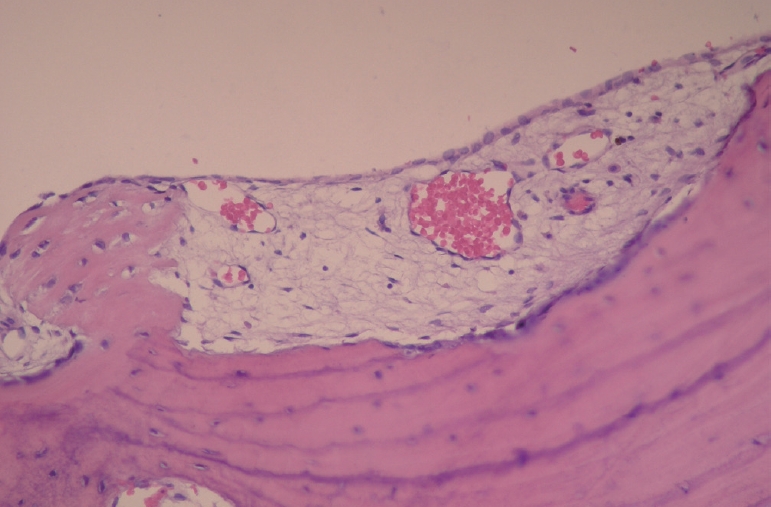
Histological examination showed normal appearing bone spicules with parts of vascular connective tissue.
Ⅲ. Discussion
TBCs in the mandible are usually located between the mandibular canine and permanent first molar. TBCs typically appear in the second decade of life. In the majority of cases, the lesion is asymptomatic and detected incidentally during a routine radiographic examination[12,17]. The two cases in this study had TBCs in the anterior region of the mandible. This area of the mandible is prone to artifacts due to the patient’s position and posture when obtaining panoramic radiographs. Therefore, diagnosing lesions in the anterior region of the mandible using panoramic radiographs has limitations. The panoramic radiographs in our cases also had artifacts: the anterior region of the mandible appeared horizontally expanded and the images were blurred.
The theory that TBCs have a traumatic origin is not convincing. In both cases presented here, there was no history of trauma. However, Blum[17] and Thoma[18] reported that trauma is the main cause of TBCs. Thoma[18] proposed that subperiosteal hematoma after traumatic injury reduces blood flow to the tissues, thereby inducing osteoclastic resorption of the adjacent bone. Although the incidence of trauma is higher in boys than girls, the gender distribution of TBCs is almost equal. In addition, the most frequently affected area in trauma cases is the anterior region of the maxilla and mandible, but TBCs are found most frequently in the posterior mandible[19]. A previous study noted a history of trauma in 17 - 70% of patients with TBCs[20]. Several other factors have also been implicated in the formation of TBCs, including decreased bone formation, an imbalance between bone formation and destruction, necrosis of fatty bone marrow due to ischemia, developmental abnormalities, bone marrow infection, cystic degeneration of a preexisting bone cyst, and chronic bone infection[21,22].
Differential diagnoses of TBCs include central giant-cell granuloma and aneurysmal bone cyst. Unlike TBCs, central giant-cell granuloma may increase the mobility of teeth, and cause root resorption and expansion of cortical plates. Histologically, an increased number of multinucleated giant cells are seen within the fibrous connective tissue and there is marked infiltration by monocytes. Conversely, aneurysmal bone cyst presents as a diffuse, firm swelling that causes teeth malocclusion and facial deformity. Histological findings include numerous sinusoidal spaces that are devoid of endothelial lining and separated by fibrous connective tissue septa[23]. Aneurysmal bone cysts are often associated with, and may originate secondary to, other osseous lesions. The most associated lesions include fibrous dysplasia, TBC, intraosseous hemangioma, and benign and malignant bone tumors[24,25].
There are two treatment options. TBC can be managed surgically, or conservatively with watchful waiting for spontaneous resolution. Several studies support long-term observation of the lesion instead of surgery[26]. Because spontaneous resolution of the TBC is more likely in younger patients, many believe that the incidence of TBCs decreases with age due to spontaneous resolution[27]. Some experts have argued that monitoring without surgery may increase the risk of complications, such as pathologic fractures. Biopsy obtained during surgery is also required to confirm the diagnosis of TBC[28-30]. The treatment for TBCs involves surgical exploration and curettage of the lesion to induce hemorrhage[4,14]. Wound healing may be improved by additional procedures such as PRF injection[31], packing the cystic cavity with absorbable gelatin (Gelfoam®, Pharmacia & Upjohn Company, Michigan, USA)[28], and adding bone graft material[29] to the surgical wound. In contrast, Precious and McFadden[32] reported that a unilocular TBC increased in size and recurred in multilocular form after curettage alone. Autogenic blood was infused into the bone during re-operation; the lesion decreased in size and bone started regenerating, as revealed by the follow-up scan.
Preparing PRF is simple and does not require anticoagulants or bovine thrombin[33]. Marelli and Tatullo[34] found that PRF, a fibrin mesh, promotes cell migration and inhibits the release of growth factors, such as vascular endothelial growth factor, platelet-derived growth factor, and transforming growth factorβ, which are essential for tissue regeneration. Concentrated growth factors induce cicatrization, angiogenesis, cellular differentiation, and osteocyte proliferation. Some studies presented that a slight increase in centrifugation time and reduction in speed resulted in better regenerative potential[35-37].
Resorbable collagen matrix induces bone regeneration and prevents the growth of soft tissues until bone is formed[38,39]. Bone graft material was not used during surgery in our cases. Autologous bone contains osteoprogenitor cells, collagenous bone matrix, bone minerals, and growth factors such as bone morphogenetic protein. However, several complications are associated with bone grafting, including pain in the donor site, infection, graft exposure, and failure of tooth eruption.
Newton and Zunt[40] emphasized the need for endodontic treatment of the adjacent teeth before or after surgery for TBC. All teeth were vital and non-tender to percussion in both of our patients, so endodontic treatment was not performed.
In this study, both patients received surgical treatment. Our patients were asymptomatic but radiographic images revealed thinning of the labiolingual cortical bone. Because the lesion is an empty cavity, it is highly vulnerable to fractures[41]. There are no standards for surgical treatment of TBC. But, many studies recommended surgical curretage because it generally results in short-term healing[42-46]. Also, surgical exploration not only confirms the diagnosis but also is curative as the curettage performed during the procedure induces bleeding and further osseous regeneration[46].
Ⅳ. Summary
TBCs appear most frequently in adolescents and do not have a gender predilection. The lesions are usually asymptomatic and are often detected incidentally during routine panoramic radiographic examination. The preliminary diagnosis of TBC was based on the clinical finding that there was no pain, facial swelling, displacement of teeth and root resorption. And the result of histological examination confirmed the diagnosis of TBC. Surgical exploration and curettage of the lesion is recommended for treating TBCs but spontaneous resolution may also occur. In this study, surgical interventions were performed on two female patients of TBC at a similar age. The surgery was performed to remove the lesion, confirm the diagnosis, rule out other diagnostic possibilities and promote wound healing. The first patient was treated with PRF and the second patient was treated with resolvable collagen matrix after curettage. A year later, follow-up radiographs confirmed healing and showed no evidence of recurrence.